Hard ground makes too great resistance, as air makes too little resistance, to the surfaces of roots.
—Jethro Tull, 1733
Under natural conditions, soils are generally stable and effectively store water, nutrients and carbon, which are cycled efficiently with plants, animals and the atmosphere. With the onset of agricultural development—as early as 10,000 years ago in western Asia and continuing today in countries such as Brazil—this balance was disrupted and soils became degraded. On sloping lands tillage generated erosion and the topsoil was washed or blown away. In many irrigated areas salts would build up and make the land unsuitable for crops. Further stress was put onto soils with increasing mechanization, heavier equipment, more intensive tillage, the export of grains and contamination from industrial products.
Soil organic matter levels are directly impacted by tilling the soil and subsequent water runoff, and by erosion. As soils are disturbed and aggregates are broken down, more soil organic matter is lost by way of making particles of organic matter more available to soil organisms. This loss of organic matter then makes the soil more susceptible to erosion. Thus a downward spiral of soil degradation commonly occurs, with the end result being lower crop yields (Figure 6.1).
Now, with increasing awareness and understanding of the causes and consequences of soil degradation, there is a need to adopt practices that reverse these trends.
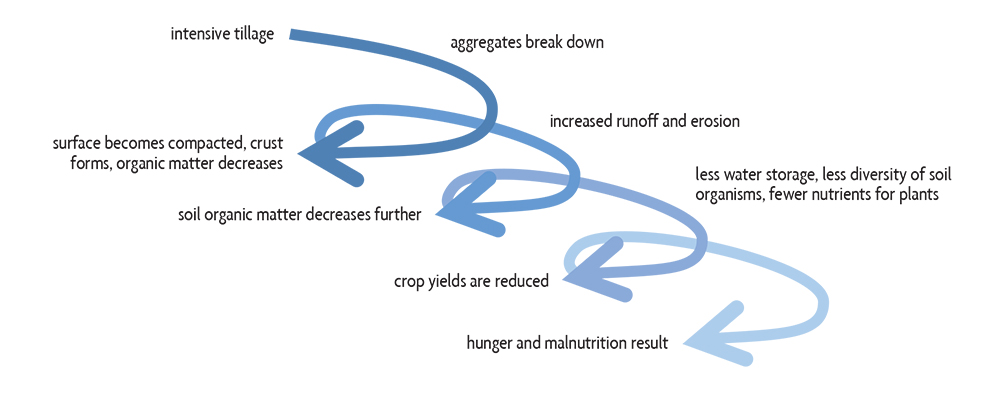
Erosion
Soil loss during agricultural production is mainly caused by water, wind and tillage. Additionally, landslides (gravitational erosion) may occur on very steep slopes. While water erosion and landslides occur under extremely wet soil conditions, wind erosion is a concern with very dry soil. Tillage erosion occurs on fields that are either steep or have undulating topography. Erosion is the result of the combination of an erosive force (water, wind or gravity), a susceptible soil and several management- or landscape-related factors. A soil’s inherent susceptibility to erosion (its erodibility) is primarily a function of its texture (generally, silts more so than sands and clays), its aggregation (the strength and size of aggregates, related to the amount of organic matter and clay), and soil water conditions. Many management practices can reduce soil erosion, although different types of erosion have different solutions.
Water Erosion
Water erosion is especially severe on bare, sloping land when intense rainfall rates cause runoff. The water flowing over the soil surface concentrates into tiny streamlets, which detach the saturated soil and transport the particles downhill. Runoff water gains more energy as it moves down the slope, scouring away more soil and also carrying more agricultural chemicals and nutrients, which end up in streams, lakes and estuaries (Figure 6.2). Erosion can involve broad areas in fields where small depths of soil are removed all the way to deep gullies that leave scars in the landscape.


Soil erosion is of greatest concern when the surface is unprotected and directly exposed to the destructive energy of raindrops and wind (Figure 6.2). The erosion process leads to a decrease in soil organic matter and aggregation, which in turn promotes further erosion. Thus, a vicious cycle begins. Soil is degraded because the most fertile part of the soil, the surface layer enriched in organic matter, is removed by erosion. Erosion also selectively removes the more easily transported finer soil mineral particles, clays, which help store nutrients and organic matter and stabilize soil aggregates. Severely eroded soils, therefore, have less favorable physical, chemical and biological characteristics, leading to a reduced ability to sustain crops and an increased potential for harmful environmental impacts.
The lower infiltration capacity of eroded soils reduces the amount of water that is available to plants and the amount that percolates through the soil into underground aquifers, while increasing the potential for flooding. This reduction in underground water recharge results in streams drying up during drought periods. Watersheds with degraded soils thus experience lower stream flow during dry seasons and increased flooding during times of high rainfall, undesirable in both cases. In fact, we surmise that the trend of increased flooding in many areas is not only the result of changed weather patterns but also compounded by gradual soil degradation.
Wind Erosion

The photograph of wind erosion from the Dust Bowl era (Figure 6.3) provides a graphic illustration of land degradation. Wind erosion can occur when soil is dry and loose, the surface is bare and smooth, and the landscape has few physical barriers to wind. The wind tends to roll and sweep larger soil particles along the soil surface, which will dislodge other soil particles and increase overall soil detachment. The smaller soil particles (very fine sand and silt) are lighter and will go into suspension in the atmosphere. They can be transported over great distances, sometimes across continents and oceans. Wind erosion affects soil quality through the loss of topsoil rich in organic matter and can cause crop damage from abrasion (Figure 6.4). In addition, wind erosion affects air quality, which is a serious concern for nearby communities. During the Dust Bowl, soil was blown all the way from the central part of the continent to New York and Washington, making East Coast residents directly aware of the environmental disaster occurring in the middle of the continent.
The ability of wind to erode a soil depends on how that soil has been managed, because strong aggregation makes it less susceptible to dispersion and transportation. In addition, many soil-building practices like no-till, mulching and the use of cover crops protect the soil surface from both wind and water erosion.
Soil and Water Conservation in Historical Times

Some ancient farming civilizations recognized soil erosion as a problem and developed effective methods for runoff and erosion control. Ancient terracing practices are apparent in various parts of the world, notably in the Andean region of South America and in Southeast Asia. Other cultures, like in pre-Columbian America, did not till the fields and effectively controlled erosion using mulching and intercropping. Some ancient desert civilizations, such as the Anasazi in the southwestern United States (600–1200 A.D.), retained runoff water and eroded silt from upper parts of the landscape with check dams to grow crops in downhill depressions (see the picture of a now forested site). For most agricultural areas of the world today, erosion still causes extensive damage (including the spread of deserts) and remains the greatest threat to agricultural sustainability and water quality.


Landslides
Landslides occur on steep slopes when the soils have become supersaturated from prolonged rains. They are especially of concern in mountainous countries where high population pressure resulted in farming on steep hillsides (Figure 6.5). The sustained rains saturate the soil, especially in landscape positions that concentrate water from upslope areas. This has two effects: It increases the weight of the soil mass (all pores are filled with water), and it decreases the cohesion of the soil (see the compaction of wet soil in Figure 6.12, right) and thereby its ability to resist the force of gravity. Agricultural areas are more susceptible than forests because they lack large, deep tree roots that can hold soil material together and may be without living vegetation for a portion of the year. Pastures on steep lands, common in many mountainous areas, typically have shallow-rooted grasses and may readily experience slumping. With certain soil types, landslides can become liquefied and turn into mudslides.
Tillage Erosion

Tillage promotes water and wind erosion by breaking down aggregates and exposing soil to the elements. But it can also cause erosion by routinely moving soil down the slope to lower areas of the field, which becomes an increasing problem with more intensive mechanized tillage. In complex topographies—such as seen in Figure 6.6—tillage erosion ultimately removes surface soil from knolls and deposits it in depressions (swales) at the bottom of slopes. What causes tillage erosion? Basically, when soil is moved by a plow or harrow on sloping land it causes more soil to move into the downslope than the upslope direction, resulting in net downslope transport. As an analogy, when throwing a ball upwards or downwards on a hillside it will go a farther distance in the down direction. Soil is similarly thrown farther downslope when tilling in the downslope direction than is thrown uphill when tilling in the upslope direction (Figure 6.7a). Over many years this has the cumulative effect of moving a lot of soil down the slope. Also, downslope tillage (with gravity) typically occurs at greater speeds than when traveling uphill (against gravity), making the situation even worse.
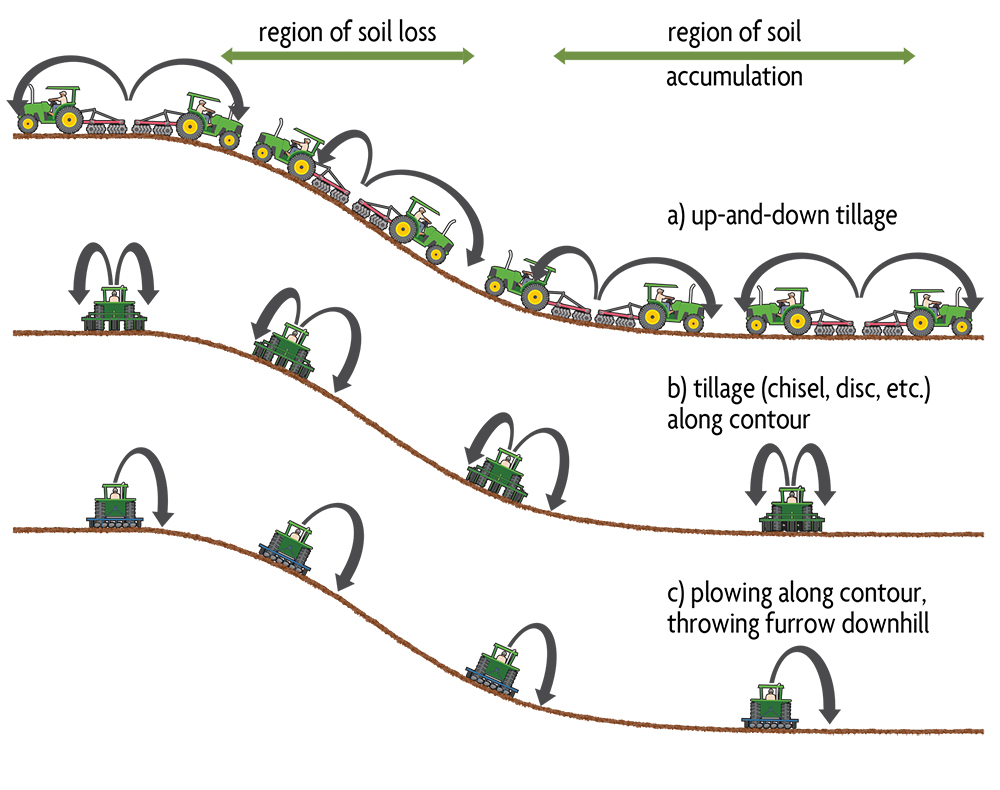
Tillage along the contour also results in downslope soil movement. Soil lifted by a tillage tool comes to rest at a slightly lower position on the slope (Figure 6.7b). A more serious situation occurs when using a moldboard plow along the contour. Moldboard plowing is often performed by throwing the soil to the side and down the slope, as this inverts the soil better than by trying to turn the furrow up the slope (Figure 6.7c).
One unique feature of tillage erosion compared to wind, water and gravitational erosion is that it is unrelated to extreme weather events and occurs gradually with every tillage operation. Tillage erosion makes field management more challenging as it results in lower crop productivity on the knolls and hillsides, and higher productivity in the swales. However, it does not generally result in offsite damage because the soil is merely moved from higher to lower positions within a field. But it is another reason to reduce tillage on sloping fields.
Soil Tilth and Compaction
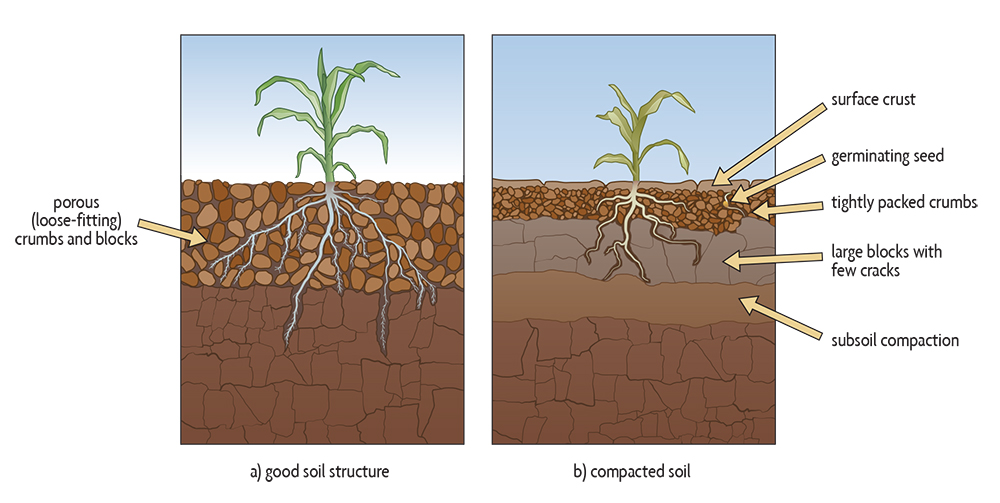
A soil becomes more compact, or dense, when aggregates or individual particles of soil are forced closer together. Soil compaction has various causes and different visible effects. It can occur either at or near the surface (shallow compaction, which includes surface crusting) or deeper down in the soil (subsoil compaction). See Figure 6.8.
Shallow Compaction
Shallow compaction, which is compaction of the surface layer or plow layer, occurs to some extent in all intensively worked agricultural soils. It is the result of a loss of soil aggregation that typically has three primary causes: erosion, reduced organic matter levels and forces exerted by the weight of field equipment. The first two result in reduced supplies of sticky binding materials and a subsequent loss of aggregation. Livestock can damage pastures through their hoof action during times when soils are susceptible to compaction.
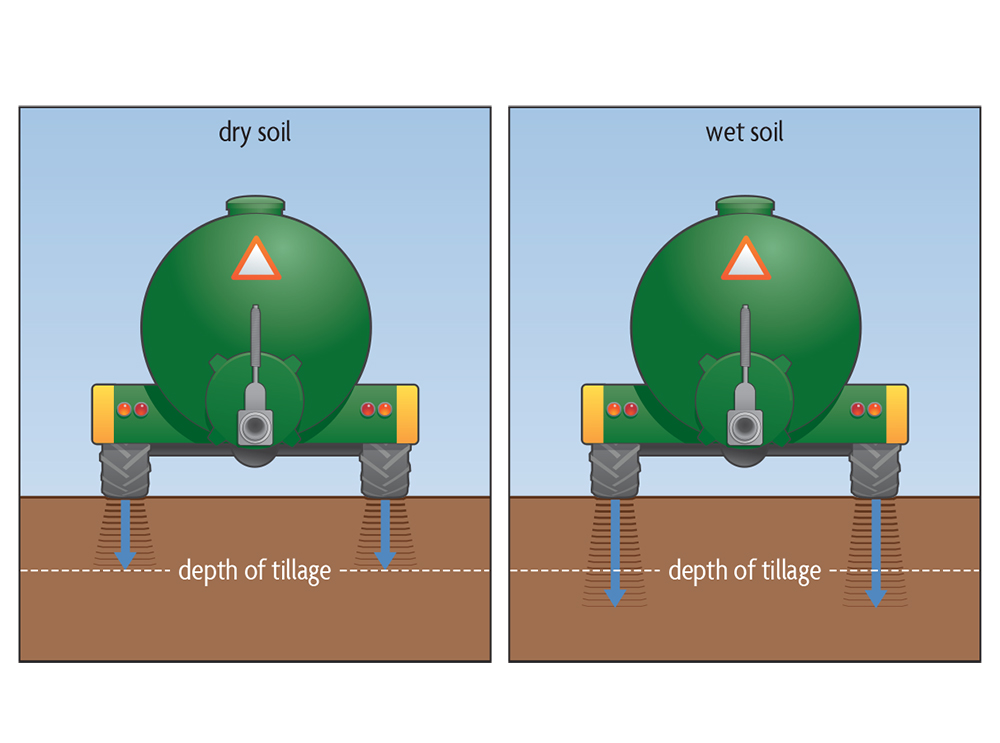
Compaction of soils by heavy equipment and tillage tools is especially damaging when soils are wet. To understand this, we need to know a little about soil consistence, or how soil reacts to external forces. At very high water contents, a soil may behave like a liquid (Figure 6.9) because it has little internal cohesion (Figure 5.10, left). On a slope it can simply flow as a result of the force of gravity, as with mudslides during excessively wet periods. At slightly lower water contents, soil has somewhat more cohesion, but it can still be easily molded and is said to be plastic (Figure 6.9). Upon further drying, the soil will become friable: it will break apart rather than mold under pressure (Figure 6.9).
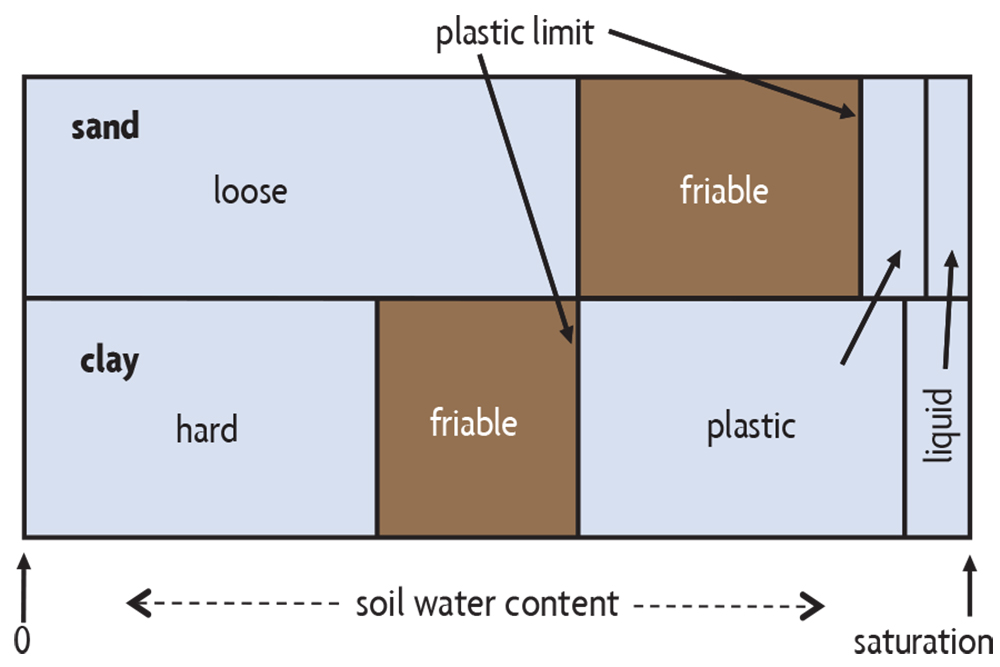
The point between plastic and friable soil, the plastic limit, has important agricultural implications. When a soil is wetter than the plastic limit, it may become seriously compacted if tilled or trafficked because soil aggregates are pushed together into a smeared, dense mass. This may be observed when you see shiny, cloddy furrows or deep tire ruts in a field (Figure 6.10). The soil is more resistant to deformation when the soil is friable (the water content is below the plastic limit). It crumbles when tilled and aggregates resist compaction by field traffic. Thus, the potential for compaction is strongly influenced by the timing of field operations, as it is much lower when the soil is adequately dry. A soil’s consistency is strongly affected by its texture (Figure 6.9). For example, as coarse-textured sandy soils drain, they rapidly change from being plastic to being friable. Fine-textured loams and clays need longer drying periods to lose enough water to become friable. This extra drying time may cause delays when scheduling field operations.
Soils are thus less susceptible to compaction when they are dry, which may be a better time to run heavier equipment. Similarly, when soils are frozen and the soil particles are fused by ice, the soil becomes solid and resistant to compaction.

Surface sealing and crusting. This problem is also caused by aggregate breakdown but specifically occurs when the soil surface is unprotected by crop residues or plant canopies. The energy of raindrops disperses wet aggregates, pounding them apart so that particles settle into a thin, dense layer. The sealing of the soil reduces water infiltration, and the surface forms a hard crust when dried. Crusting generally occurs after tillage and planting when the soil is unprotected, and it can delay or prevent seedling emergence. Even when the crust is not severe enough to limit germination, it can reduce water infiltration. Soils with surface crusts are prone to high rates of runoff and erosion. You can reduce surface crusting by leaving more residue on the surface and by maintaining strong soil aggregation. Sometimes, farmers break crusts with a harrow, but that only treats the symptom, not the cause.
Intensive tillage. Shallow compaction is especially common with repeated soil disturbance. Tillage operations often become part of a vicious cycle in which a compacted soil tills up very cloddy (Figure 6.11a) and then requires extensive secondary tillage and packing trips to create a satisfactory seedbed (Figure 6.11b). Natural aggregates break down, and organic matter decomposes in the process—contributing to more compaction in the future. Although the final seedbed may be ideal at the time of planting, rainfall shortly after planting may cause surface sealing and further settling (Figure 6.11c) because few sturdy aggregates are present to prevent the soil from dispersing. The result may be a dense soil with a crust at the surface. Some soils may hard-set like cement, even after the slightest drying, thereby slowing plant growth. Although the soil becomes softer when it re-wets, that moisture provides only temporary relief to plants.
Subsoil Compaction
Subsoil compaction occurs deeper in the soil and is sometimes referred to as a plow pan, although it is commonly caused by more than just plowing. Subsoil is prone to compaction because it is usually wetter, denser, higher in clay content, lower in organic matter, and less aggregated than topsoil. Also, subsoil is not loosened by regular tillage and cannot easily be amended with additions of organic materials. Another challenge is that the subsoil is by definition buried and therefore compaction is invisible unless you dig down or push a rod into the soil.Subsoil compaction occurs when farmers run heavy vehicles, especially those with poor weight distribution. The load exerted on the surface is transferred into the soil along a cone-shaped pattern (Figure 6.12). With increasing depth, the compaction force is distributed over a larger area, thereby reducing the pressure in deeper layers. When the loading force at the surface is small, say through foot or hoof traffic or a light tractor, the pressure exerted deep in the soil is minimal. But when the load is high from heavy equipment, like with a heavy manure spreader or combine, the pressures at depth are sufficient to cause considerable soil compaction. When the soil is wet, the force causing compaction near the surface is more easily transferred to the subsoil, which causes even more compaction damage. Clearly, the most severe compaction in subsoils occurs with the combination of heavy vehicle traffic and wet soil conditions.
Check Before Tilling
To be sure that a soil is ready for equipment use, you can do the simple “ball test” by taking a handful of soil from the lower part of the plow layer and trying to make a ball out of it. If it molds easily and sticks together, the soil is too wet. If it crumbles readily, it is sufficiently dry for tillage or heavy traffic.
Another major cause of subsoil compaction is the pressure of a tillage implement, especially a plow or disk, pressing on the soil below (hence the term plow pan).
Plows cause compaction because the weight of the plow plus the lifting of the furrow slices results in high downward forces from the plow share (bottom) onto the soil layer immediately underneath. Disks also have much of their weight concentrated at the bottom of the disk and can cause shallow pans. Subsoil compaction may also occur during moldboard plowing when a set of tractor wheels is placed in the open furrow, thereby applying wheel pressure directly to the soil below the plow layer. Overall, these pans are very common in soil that has been plowed, sometimes even many years after the field was converted to no-till.



Consequences of Compaction
As compaction pushes particles closer together, the soil becomes dense and pore space is lost. Notably, the large pores are lost as they are compressed into smaller ones (Figure 6.13). Loss of large pores between aggregates is particularly harmful for fine- and medium-textured soils that depend on those pores for good infiltration and percolation of water, as well as air exchange with the atmosphere. Although compaction can also damage coarse-textured soils, the impact is less severe. They depend less on aggregation because the pores between individual particles are sufficiently large to allow good water and air movement.


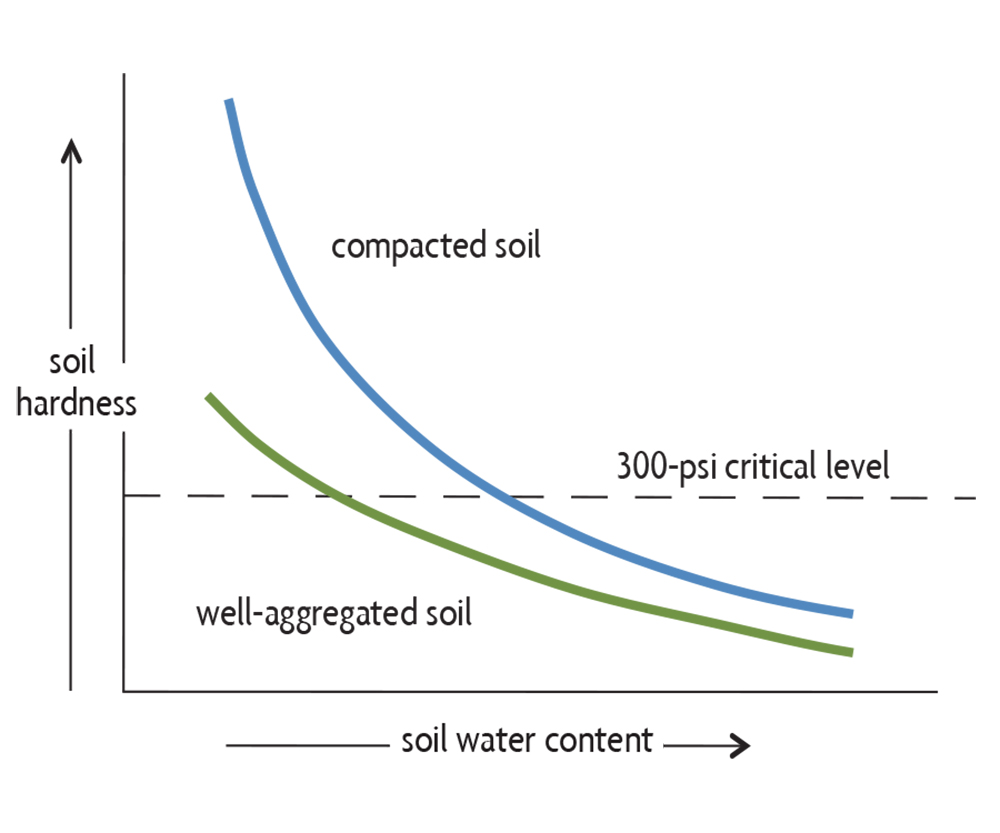
Compacted soil becomes hard when it dries, as it has many small pores that can hold water under high suction and pull particles tightly together. This can restrict root growth and the activity of soil organisms. Compacted soils typically have greater resistance to penetration at a given soil moisture level than a well-structured soil (Figure 6.14), which has large pores between aggregates that therefore easily pull apart. The resistance to penetration for a moist, high-quality soil is usually well below the critical level where root growth ceases for most crops: 300 pounds per square inch (psi, or 2 megapascals). As the soil dries, its strength increases, but a high-quality soil may not exceed the critical level for most (or all) of the moisture range. A compacted soil, on the other hand, has a very narrow water content range for good root growth. The soil has increased resistance to penetration even in the wet range (the soil is hard). When it dries, a compacted soil hardens quicker than a well-structured soil, rapidly becoming so hard that it is well above the critical 300-psi level that restricts root growth.
Some Crops Are More Sensitive Than Others
Compaction doesn’t affect all crops to the same extent. An experiment in New York found that direct-seeded cabbage and snap beans were more harmed by compaction than were cucumbers, table beets, sweet corn and transplanted cabbage. Much of the plant damage was caused by the secondary effects of compaction, such as prolonged soil saturation after rain, reduced nutrient availability or uptake, and greater pest susceptibility. Some crops also grow more roots when the soil is soft. For example, cool-season crops that grow well in the early season can take advantage of moister, softer soil conditions, while summer crops may experience dryer, harder soils.
Restricted Rooting
Actively growing roots need large pores with diameters greater than about 0.1 millimeter, the size of most root tips. Roots must enter the pore and anchor themselves before continuing growth. Compacted soils that have few or no large pores don’t allow plants to be effectively rooted, thus limiting water and nutrient uptake.
What happens when root growth is limited? The root system will probably develop short, thick roots and few fine roots or root hairs (Figure 5.6, Figure 6.8). The few thick roots may be able to find some weak zones in the soil, often by following crooked patterns. These roots have thickened tissue and are not efficient at taking up water and nutrients. In many cases, roots in degraded soils do not grow below the surface layer into the subsoil (see Figure 6.8); it’s just too dense and hard for them to grow. Deeper root penetration is especially critical under rain-fed agriculture. The limitation on deep root growth by subsoil compaction reduces the volume of soil from which plant roots can extract water and nutrients, and increases the probability of yield losses from drought stress.
There is also a more direct effect on plant growth, beyond the reduced soil volume for roots to explore. A root system that’s up against mechanical barriers sends a hormonal signal to the plant shoot, which then slows down respiration and growth. This plant response appears to be a natural survival mechanism similar to what occurs when plants experience water stress. In fact, because some of the same hormones are involved—and mechanical resistance increases when the soil dries—it is often difficult to separate the effects of compaction from those of drought.
We have learned much about the effects of compaction on root growth, but we know less about the effects on soil organisms. However, it is well established that a diverse soil ecosystem requires organisms to have spaces for habitation and movement. Earthworms and insects, for example, need large pores to move around and access organic materials, while aerobic bacteria and fungi need air exchange. Therefore, compacted soils typically have much lower populations of these beneficial organisms, but they return remarkably quickly when better practices are adopted.
The Water Range for Best Plant Growth
The limitations to plant growth caused by compaction and water extremes can be combined into the concept of the optimum water range for plant growth: the range of water contents under which plant growth is not reduced by drought, mechanical stress or lack of aeration (Figure 6.15). This range, referred to by scientists as the least-limiting water range, is bounded on two sides: when the soil is too wet and when it’s too dry.
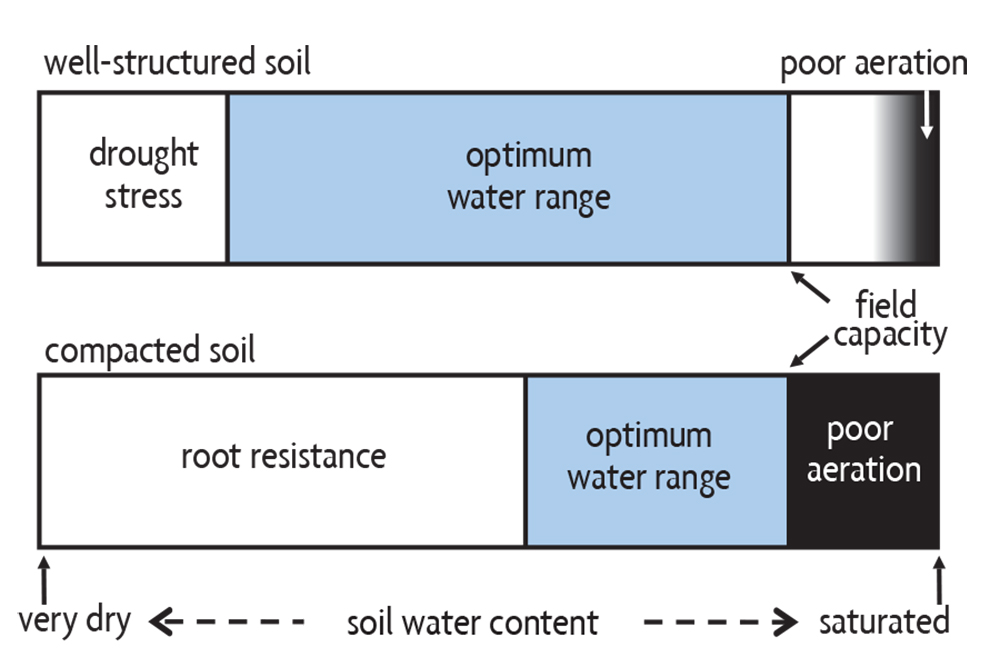
The optimum water range in a well-structured soil has its field capacity on the wet end, as water above that moisture content is quickly drained out by gravity. On the dry end is the wilting point, beyond which the soil holds water too tightly to be used by plants. However, the soil water range for best growth in a compacted soil is much narrower. Even after a severely compacted soil drains to field capacity, it is still too wet because it lacks large pores and is thus poorly aerated. Good aeration requires at least 20% of the pore space (about 10% of the volume of the whole soil) to be air filled. On the dry end, plant growth in a compacted soil is commonly limited by soil hardness rather than by lack of available water. Plants in compacted soils therefore experience more stress during both wet and dry periods than plants in soils with good tilth. The effects of compaction on crop yields usually depend on the length and severity of excessive wet or dry periods and when those periods occur relative to critical times for plant growth.
Chemical Contamination of Soil
Soils can be contaminated with chemicals, either naturally or by human activity, to such an extent that crops are adversely affected. Problems of saline and sodic (alkaline) soils are most found in arid and semiarid regions, or in soil affected by coastal flooding. Other types of contamination may derive from natural toxic chemicals or pollution.
Saline and Sodic Soils
Special soil problems are found in arid and semiarid regions, including soils that are high in salts, called saline soils, and those that have excessive sodium (Na+), called sodic soils. Sometimes these go together and the result is a saline-sodic soil. Saline soils usually have good soil tilth, but plants can’t get the water they need because the high salt levels in the soil inhibit water uptake as the soil exerts an osmotic force that counters the plant’s own osmotic potential.
Saline Soil
Electrical conductivity of a soil extract is greater than 4 ds/m, enough to harm sensitive crops.
Sodic Soil
Sodium occupies more than 15% of the cation exchange capacity (CEC). Soil structure can significantly deteriorate in some soils at even lower levels of sodium.
Sodic soils tend to have very poor physical structure because the high sodium levels cause clays to disperse, leading aggregates to break apart. Aggregates of sodic soils disperse when they are saturated, and the solids then settle as individual particles and make the soil very dense (Figure 6.16). These soils become difficult to work with and are very inhospitable for plants because of both compaction and greatly reduced aeration. When a sodic soil is fine textured its consistency and appearance are something like that of chocolate pudding. It causes serious problems with drainage, seedling emergence and root development. A soil like that must be remediated before growing crops.

Also, the ionic strength of the cations in the soil can affect aggregate stability. Some believe that soils with high magnesium-over-calcium ratios tend to have weaker aggregates and would benefit from calcium applications, but that has limited support from research except in unusual situations.
Saline and sodic soils are commonly found in the semiarid and arid regions of the western United States and in similar climate zones in many countries around the world. They are difficult to remediate. After major hurricanes, coastal flooding areas may also experience temporary saline-sodic conditions until the salts are washed out by rains.
Although some soils are naturally saline, sodic, or both, there are a number of ways that surface soils may become contaminated with salts and sodium. When irrigation water containing significant salt content is used without applying extra water to leach out the salts, accumulation of salts can create salinity. Also, routine use of irrigation water with high sodium levels relative to calcium and magnesium will create a sodic soil over time. Over-irrigating, which often occurs with conventional flood or furrow irrigation, can create salinity problems in the topsoil by raising water tables to within 2–3 feet of the surface. Shallow groundwater can then be wicked up by capillary action to the surface, where the water evaporates and the salts remain. Sometimes the extra moisture accumulated during a fallow year in semiarid regions causes field seeps, in which salty water high in sodium comes to the surface, leading to the development of saline and sodic patches.
Salt Presence In All Soils
Salts of calcium, magnesium, potassium and other cations, along with the common negatively charged anions chloride, nitrate, sulfate and phosphate, are found in all soils. However, in soils in humid and subhumid climates, with from 1–2 to well over 7 inches of water percolating beneath the root zone every year, salts don’t usually accumulate to levels where they can be harmful to plants. Even when high rates of fertilizers are used, salts usually become a problem only when you place large amounts in direct contact with seeds or growing plants. Salt problems also frequently occur in greenhouse potting mixes because growers regularly irrigate their greenhouse plants with water containing fertilizers and may not add enough water to leach the accumulating salts out of the pot.
Other Types of Chemical Contamination
Soils can become contaminated with many sorts of chemicals from oil, gasoline or pesticides to a variety of industrial chemicals and mining wastes. This contamination may occur through unintended spills, but in the past, waste materials were often deliberately disposed of by dumping on fields. In urban areas it is common to find lead-contaminated soils as a result of the past use of lead-based gasoline and paint. Lead, as well as other contaminants, frequently makes creating an urban garden a real challenge. Often, new topsoil is brought in, mixed with a large quantity of compost, and placed in raised beds so that plant roots grow above the contaminated soil, and the lead is made less available by organic chelates. Agricultural soils that have a history of applications of sewage sludge (biosolids is the current term) may have received significant quantities of heavy metals such as cadmium, zinc and chromium, as well as antibiotics, pharmaceutical drugs and an assortment of toxic organic chemicals contained in the sludge. Some phosphorus fertilizers contain cadmium that can build up in soils. Toxicity related to such contaminants may impact plants and humans, like itai-itai disease among Japanese rice growers in the 1950s.
There are a number of ways to remediate chemically contaminated soils. Sometimes adding manure or other organic amendments and growing crops stimulates soil organisms to break down organic chemicals into less harmless forms. For example, pesticides, organic wastes and oils can be naturally broken down in soils. Some plants are especially good at taking up certain metals from soil and can be used to clean contaminated soil (but they then must be disposed of carefully). Adding organic matter can also reduce the availability of heavy metals by forming chelates (Figure 2.5).
Urban Soils
Severe soil degradation can be observed in urban areas where soil is often intensively used, physically disturbed or contaminated by a wide variety of chemicals. In addition, urban ecosystems are challenged by a difficult microclimate (so-called heat islands). On the positive side, urban spaces are very valuable—many people use them—and there are therefore more financial resources available to invest in remediation. Also, urban areas concentrate organic materials that can be used for soil improvement, like food waste and street leaves turned into compost to help build soil organic matter, and urban tree branches that are chipped and used for mulch. For more on the special issues of growing plants on urban soils see Chapter 22.
Chapter 6 Summary
Soil degradation is one of the world’s great environmental problems. At the same time as rivers are contaminated with sediments eroded from soils, severe erosion in many parts of the world results in a significant decrease in soil productivity. Although the immediate cause for water erosion may be intense rainfall, there are a number of reasons soil loss is especially severe in some situations. Compaction, another form of soil degradation, can go unnoticed unless one looks for the symptoms, but it can have a damaging effect on plant growth. Chemical contamination, whether from salts, metals or organics can also affect plant and human health. Many of these concerns can be addressed through good management practices. For a discussion of tried and true ways of reducing erosion and compaction, see chapters 14 and 15. For how to reclaim saline, sodic and saline-sodic soils, see Chapter 20.
Chapter 6 Sources
Dangour, A.D., K. Lock, A. Hayter, A. Aikenhead, E. Allen and R. Uauy. 2010. Nutrition-related health effects of organic foods: a systematic review. Am. J. Clinical Nutrition 92: 203–210.
da Silva, A.P., B.D. Kay and E. Perfect. 1994. Characterization of the least limiting water range of soils. Soil Science Society of America Journal 58: 1775–1781.
Letey, J. 1985. Relationship between soil physical properties and crop production. Advances in Soil Science 1: 277–294.
Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA). 1997. Soil Management. Best Management Practices Series. Available from the Ontario Federation of Agriculture, Toronto, Ontario, Canada.
Roberts, T. 2014. Cadmium and phosphorous fertilizers: The issue and the science. Procedia Engineering 83: 52–59.
Seufert, V. and N. Ramankutty. 2017. Many shades of gray—the context-dependent performance of organic agriculture. Science Advances. 2017; 3: e1602638.
Soehne, W. 1958. Fundamentals of pressure distribution and soil compaction under tractor tires. Agricultural Engineering 39: 276–282.
Tull, J. 1733. The horse-hoeing husbandry: Or an essay on the principles of tillage and vegetation. Printed by A. Rhames, for Gunne, G. Risk, G. Ewing, W. Smith, & Smith and Bruce, Booksellers. Available online through the Core Historical Literature of Agriculture, Albert R. Mann Library, Cornell University, http://chla.library.cornell.edu. Unger, P.W. and T.C. Kaspar. 1994. Soil compaction and root growth: A review. Agronomy Journal 86: 759–766.
