Follow the appropriateness of the season, consider well the nature and conditions of the soil, then and only then least labor will bring best success. Rely on one’s own idea and not on the orders of nature, then every effort will be futile.
—Jia Sixie, 6th century, China
As we will discuss at the end of this chapter, organic matter has an overwhelming effect on almost all soil properties, although it is generally present in relatively small amounts. A typical agricultural soil has 1–6% organic matter by weight. It consists of three distinctly different parts: living organisms, fresh residues and molecules derived from well-decomposed residues. These three parts of soil organic matter have been described as the living, the dead and the very dead. This three-way classification may seem simple and unscientific, but it is very useful in understanding soil organic matter.
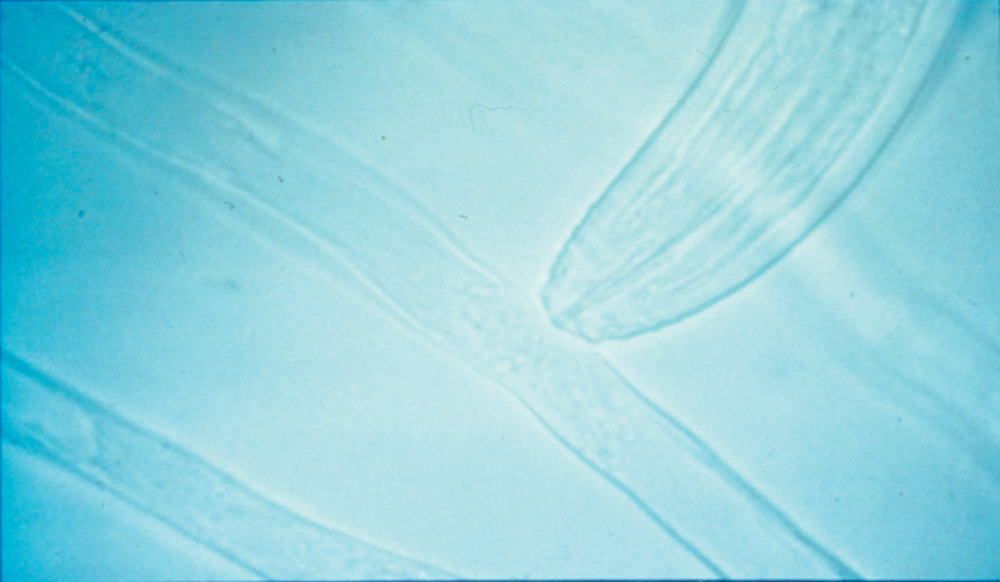
The living. This part of soil organic matter includes a wide variety of microorganisms, such as bacteria, viruses, fungi, protozoa and algae. It even includes plant roots and the insects, earthworms and larger animals, such as moles, woodchucks and rabbits that spend some of their time in the soil. The living portion represents about 15% of the total soil organic matter. The range of organisms in soil is so great that it is estimated that they represent about 25% of the world’s total biodiversity. Microorganisms, earthworms and insects feed on plant residues and manures for energy and nutrition, and in the process they mix organic matter into the mineral soil. In addition, they recycle plant nutrients. Sticky substances on the skin of earthworms and other materials produced by fungi help bind particles together. This helps to stabilize the soil aggregates, which are clumps of particles that make up good soil structure. Sticky substances on plant roots as well as the proliferation of fine roots and their associated mycorrhizae help promote development of stable soil aggregates. Organisms such as earthworms and some fungi also help to stabilize the soil’s structure (for example, by producing channels that allow water to infiltrate) and, thereby, improve soil water status and aeration. Plant roots also interact in significant ways with the various microorganisms and animals living in the soil. Another important aspect of soil organisms is that they are in a constant struggle with each other (Figure 2.1). Further discussion of the interactions between soil organisms and roots, and among the various soil organisms, is provided in Chapter 4.
A multitude of microorganisms, earthworms and insects get their energy and nutrients by breaking down organic residues in soils. At the same time, much of the energy stored in residues is used by organisms to make new chemicals as well as new cells. How does energy get stored inside organic residues in the first place? Green plants use the energy of sunlight to link carbon atoms together into larger molecules. This process, known as photosynthesis, is used by plants to store energy for respiration and growth, and much of this energy ends up as residues in the soil after the plant dies.
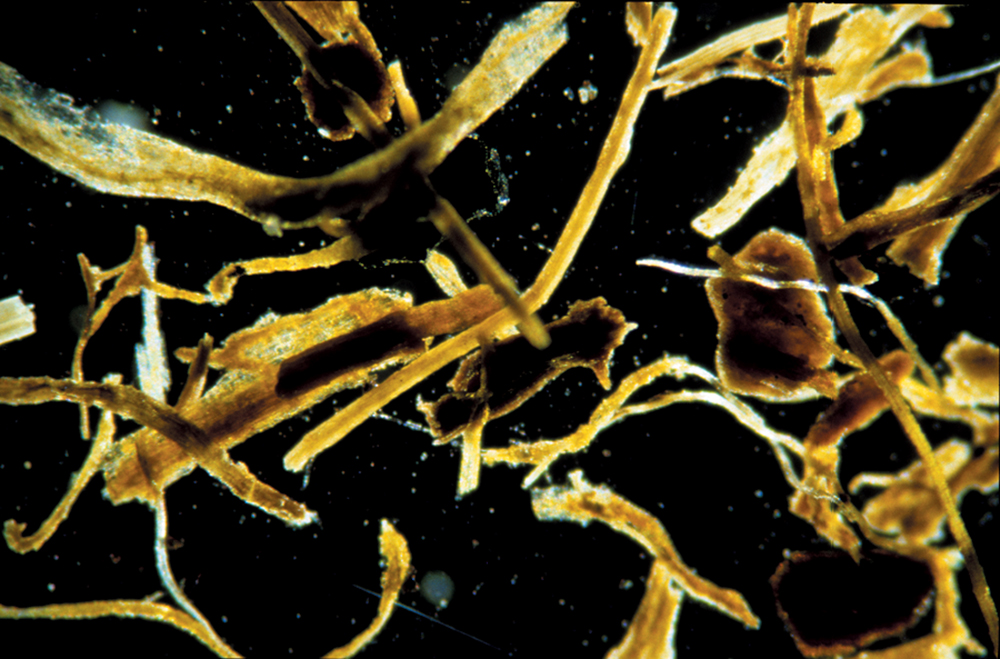
The dead. The fresh residues, or “dead” organic matter, consist of recently deceased microorganisms, insects, earthworms, old plant roots, crop residues and recently added manures. In some cases, just looking at them is enough to identify the origin of the fresh residues (Figure 2.2). This part of soil organic matter is the active, or easily decomposed, fraction. This active fraction of soil organic matter is the main supply of food for various organisms—microorganisms, insects and earthworms—living in the soil. As organic materials are decomposed by the “living,” they release many of the nutrients needed by plants. Organic chemical compounds produced during the decomposition of fresh residues also help to bind soil particles together and give the soil good structure.
Some organic molecules directly released from cells of fresh residues, such as proteins, amino acids, sugars and starches, are also considered part of this fresh organic matter. These molecules generally do not last long in the soil. Their structure makes them easy to decompose because so many microorganisms use them as food. Some cellular molecules such as lignin are decomposed, but it takes longer for organisms to do so. This can make up a large fraction of the soil organic matter in poorly drained soils, like peats and mucks, as well as wetlands that have been taken into agricultural production. These hold large amounts of organic matter that was not decomposed due to waterlogging, but they don’t provide the same benefits as the fresh residues.
The very dead. This includes other organic substances in soils that are difficult for organisms to decompose. Some use the term humus to describe all soil organic matter. We’ll use the term to refer only to that relatively stable portion of soil organic matter that resists decomposition. Humus is protected from decomposition mainly because its chemical structure makes it hard for soil organisms to utilize.
Identifiable fragments of undecomposed or partially decomposed residue, including remains of microorganisms, can be held inside aggregates in spaces too small for organisms to access. In a sense they behave as if they were “very dead” because of being inaccessible to organisms. As long as organic residue is physically protected from attack by microorganisms it will behave as part of the “very dead.” When these aggregates are broken up by freezing and thawing, drying and rewetting, or by tillage, entrapped organic fragments and simple organic substances adsorbed on clays can be made accessible to microorganisms and are readily decomposed. Because much of soil organic matter is so well protected from decomposition, physically and chemically, its age in soils can be as high as hundreds of years.
But even though humus is protected from decomposition, its chemical and physical properties make it an important part of the soil. Humus holds on to some essential nutrients and stores them for slow release to plants. Some medium-size molecules also can surround certain potentially harmful chemicals, like heavy metals and pesticides, and prevent them from causing damage to plants and the environment. The same types of molecules can also make certain essential nutrients more available to plants. Good amounts of soil humus and fragments of crop residues can lessen drainage and compaction problems that occur in clay soils. They also improve water retention in sandy soils by enhancing aggregation, which reduces soil density, and by holding on to and releasing water.
Char. Another type of organic matter, one that has gained a lot of attention lately, is usually referred to as black carbon or char. Many soils contain some small pieces of charcoal, the result of past fires of natural or human origin. Some, such as the black soils of Saskatchewan, Canada, may have relatively high amounts of char, presumably from naturally occurring prairie fires. However, an increased interest in charcoal in soils has come about mainly through the study of the soils called dark earths, the terra preta de indio that are on sites of long-occupied villages in the Amazon region of South America that were depopulated during the colonial era. These dark earths contain 10–20% black carbon in the surface foot of soil, which gives them a much darker color than the surrounding soils. The soil charcoal was the result of centuries of cooking fires and in-field burning of crop residues and other organic materials. The manner in which the burning occurred—slow burns, perhaps because of the wet conditions common in the Amazon—produced a lot of char material and not as much ash as occurs with more complete burning at higher temperatures. These soils were intensively used in the past but have been abandoned for centuries. Still, they remain much more fertile than the surrounding soils, partially due to the high inputs of nutrients in animal and plant residue that were initially derived from the nearby forest, and they yield better crops than surrounding soils typical of the tropical forest. Part of this higher fertility—the ability to supply plants with nutrients with very low amounts of leaching loss—has been attributed to the large amount of black carbon and the high amount of biological activity in the soils (even centuries after abandonment). Charcoal is a very stable form of carbon that helps maintain relatively high cation exchange capacity and supports biological activity by providing suitable habitat. However, char does not provide soil organisms with readily available food sources as do fresh residues and compost. People are experimenting with adding biochar to soils, but this is likely not economical at large scales. The quantity needed to make a major difference to a soil is apparently huge— many tons per acre—and may limit the usefulness of this practice to small plots of land, gardens and container plants, or as a targeted additive coating seeds. Also, benefits from adding biochar should be considered in comparison to what might be gained when using the same source materials like wood chips, crop residues or food waste added directly to the soil, after composting or even after complete combustion as ash.
BIOCHAR AS A SOIL AMENDMENT
It is believed that the unusually productive “dark earth” soils of the Brazilian Amazon region and other places in the world were produced and stabilized by long-term incorporation of charcoal. Black carbon, produced by wildfires as well as by human activity and found in many soils around the world, is a result of burning biomass at around 600–900 degrees Fahrenheit under low oxygen conditions. This incomplete combustion results in about half or more of the carbon in the original material being retained as char. The char, also containing ash, tends to have high amounts of negative charge (cation exchange capacity), has a liming effect on soil, retains some nutrients from the wood or other residue that was burned, stimulates microorganism populations, and is very stable in soils. Although many times increases in yield have been reported following biochar application—probably partially a result of increased nutrient availability or increased pH—sometimes yields suffer. Legumes do particularly well with biochar additions, while grasses frequently become nitrogen deficient, indicating that nitrogen may be deficient for a period following application.
Biochar is a variable material because a variety of organic materials and burn methods can be used to produce it, perhaps contributing to its inconsistent effects on soil and plants. The economic and environmental effects of making and using biochar depend on the source of organic material being converted to biochar, whether heat and gases produced in the process are utilized or just allowed to dissipate, the amount of available oxygen during biochar production, and the distance from where it is produced to the field where it is applied. On the other hand, when used as a seed coating, much less biochar is needed per acre, and it may still stimulate seedling growth and development.
Note: The effects of biochar on raising soil pH and immediately increasing calcium, potassium, magnesium, etc., are probably mostly a result of the ash rather than the black carbon itself. These effects can also be obtained by using more completely burned material, which contains more ash and little black carbon.
Carbon and organic matter. Soil carbon is sometimes used as a synonym for organic matter, although the latter also includes nutrients and other chemical elements. Because carbon is the main building block of all organic molecules, the amount in a soil is strongly related to the total amount of all the organic matter: the living organisms plus fresh residues plus well-decomposed residues. When people talk about soil carbon instead of organic matter, they are usually referring to organic carbon, or the amount of carbon in organic molecules in the soil. The amount of organic matter in soils is about twice the organic carbon level. However, in many soils in glaciated areas and semiarid regions it is common to have another form of carbon in soils—limestone, either as round concretions or dispersed evenly throughout the soil. Lime is calcium carbonate, which contains calcium, carbon and oxygen. This is an inorganic (mineral) form of carbon. Even in humid climates, when limestone is found very close to the surface, some may be present in the soil. In those cases the total amount of soil carbon includes both inorganic and organic carbon, and the organic matter content could not be estimated simply by doubling the total carbon percent. Normal organic matter decomposition that takes place in soil is a process that is similar to the burning of wood in a stove. When burning wood reaches a certain temperature, the carbon in the wood combines with oxygen from the air and forms carbon dioxide. As this occurs, the energy stored in the carbon-containing chemicals in the wood is released as heat in a process called oxidation. The biological world, including humans, animals and microorganisms, also makes use of the energy inside carbon-containing molecules. This process of converting sugars, starches and other compounds into a directly usable form of energy is also a type of oxidation. We usually call it respiration. Oxygen is used, and carbon dioxide and heat are given off in the process.
Why Soil Organic Matter Is So Important
A fertile and healthy soil is the basis for healthy plants, animals and humans. And soil organic matter is the very foundation for healthy and productive soils. Understanding the role of organic matter in maintaining a healthy soil is essential for developing ecologically sound agricultural practices. But how can organic matter, which only makes up a small percentage of most soils, be so important that we devote the three chapters in this section to discuss it? The reason is that organic matter positively influences, or modifies the effect of, essentially all soil properties, and it is what makes the soil fertile. That is the reason it’s so important to our understanding of soil health and of how to manage soils better. Organic matter is essentially the heart of the story, but, as we will discuss later, certainly not the only part. In addition to functioning in a large number of key roles that promote soil processes and crop growth, soil organic matter is a critical part of a number of global and regional cycles.
It’s true that you can grow plants on soils with little organic matter. In fact, you don’t need to have any soil at all. Although gravel and sand hydroponic systems, and even aeroponics (where a nutrient solution is sprayed directly on plant roots) without soil, can grow excellent crops, large-scale systems of this type may have ecological problems and make sense economically only for a limited number of high-value crops grown close to their markets. It’s also true that there are other important issues aside from organic matter when considering the health of a soil. However, as soil organic matter decreases, it becomes increasingly difficult to grow plants, because problems with fertility, water availability, compaction, erosion, parasites, diseases and insects become more common. Ever higher levels of inputs—fertilizers, irrigation water, pesticides and machinery—are required to maintain yields in the face of organic matter depletion. But if attention is paid to proper organic matter management, the soil can support a good crop with less need for expensive fixes.
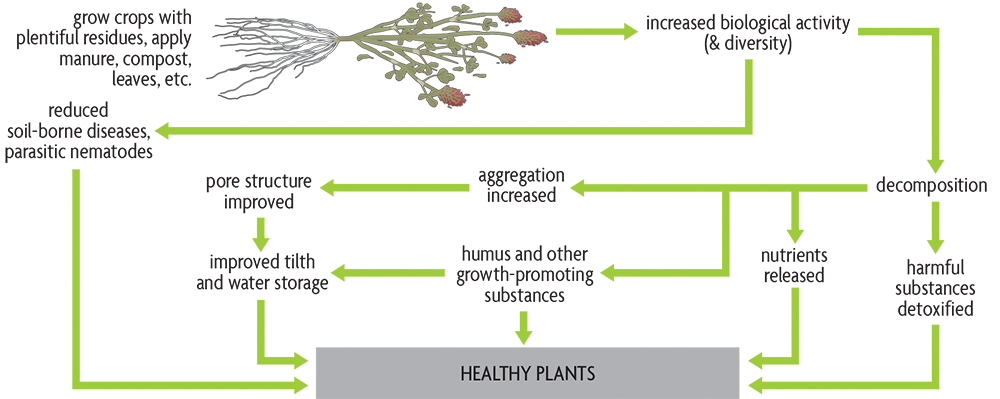
The organic matter content of agricultural topsoil is usually in the range of 1–6%. A study of soils in Michigan demonstrated potential crop-yield increases of about 12% for every 1% increase in organic matter. In a Maryland experiment, researchers saw an increase of approximately 80 bushels of corn per acre when organic matter increased from 0.8% to 2%. The enormous influence of organic matter on so many of the soil’s properties—biological, chemical and physical—makes it of critical importance to healthy soils (Figure 2.3). Part of the explanation for this influence is the small particle size of the well-decomposed portion of organic matter, the humus. Its large surface area–to–volume ratio means that humus is in contact with a considerable portion of the soil. The intimate contact of humus with the rest of the soil allows many reactions, such as the release of available nutrients into the soil water, to occur rapidly. However, the many roles of living organisms make soil life an essential part of the organic matter story.
Plant Nutrition
Plants need 17 chemical elements for their growth: carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), boron (B), zinc (Zn), molybdenum (Mo), nickel (Ni), copper (Cu), cobalt (Co), and chlorine (Cl). Plants obtain carbon as carbon dioxide (CO2) from the atmosphere (with some of that diffusing up from the soil underneath as organisms decompose organic substances). Oxygen is also mostly taken from the air as oxygen gas (O2). The remaining essential elements are obtained mainly from the soil. The availability of these nutrients is influenced either directly or indirectly by the presence of organic matter. The elements needed in large amounts—carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium and sulfur—are called macronutrients. The other elements, called micronutrients, are essential elements needed in small amounts. Sodium (Na) and silica (Si) help many plants grow better but are not considered essential to plant growth and reproduction.
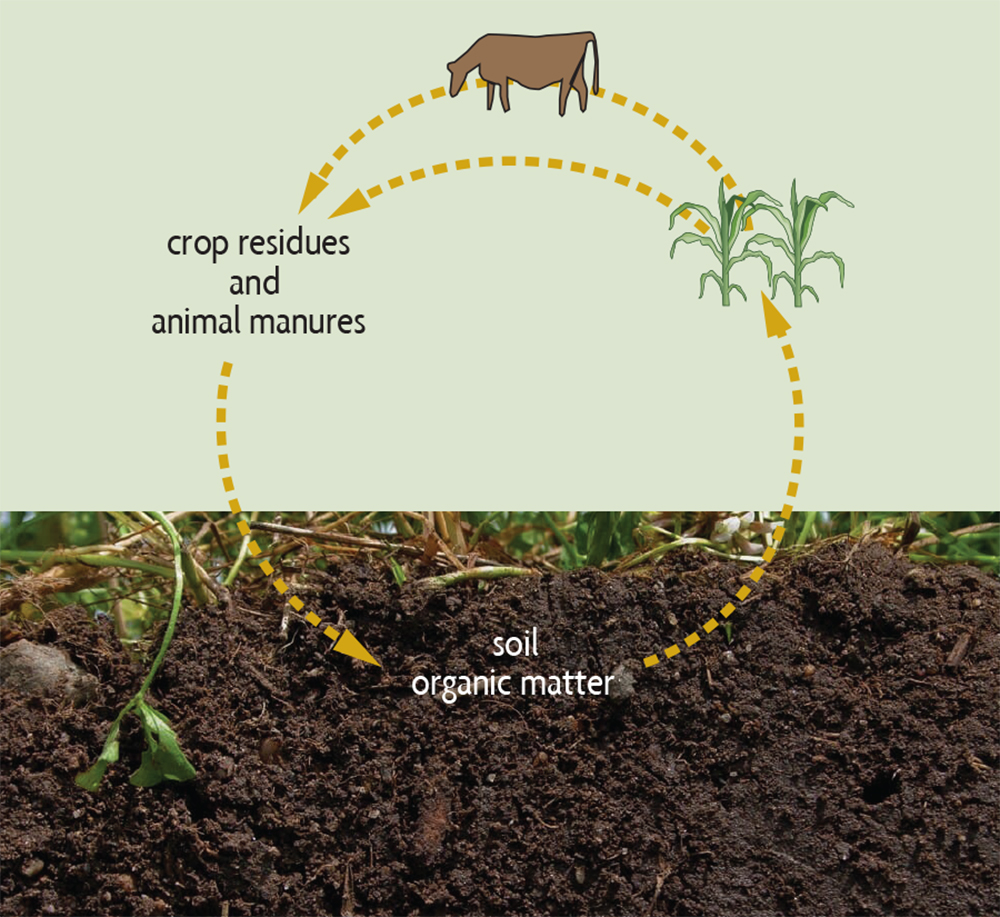
Nutrients from decomposing organic matter. Most of the nutrients in soil organic matter can’t be used by plants as long as those nutrients exist as part of large organic molecules. As soil organisms decompose organic matter, nutrients are converted into simpler, inorganic (mineral) forms that plants can easily use. This process, called mineralization, provides much of the nitrogen that plants need by converting it from organic forms. For example, proteins are converted to ammonium (NH4+) and then to nitrate (NO3–). Most plants will take up the majority of their nitrogen from soils in the form of nitrate. The mineralization of organic matter is also an important mechanism for supplying plants with such nutrients as phosphorus and sulfur, and most of the micronutrients. This release of nutrients from organic matter by mineralization is part of a larger agricultural nutrient cycle (see Figure 2.4 and Chapter 7).
WHAT MAKES TOPSOIL?
Having a good amount of topsoil is important. But what gives topsoil its beneficial characteristics? Is it because it’s on TOP? If we bring in a bulldozer and scrape off one foot of soil, will the exposed subsoil now be topsoil because it’s on the surface? Of course, everyone knows that there’s more to topsoil than its location on the soil surface. Most of the properties we associate with topsoil—good nutrient supply, tilth, drainage, aeration, water storage, etc.—are there because topsoil is rich in organic matter and contains a huge diversity of life. These characteristics diminish the farther down you dig, making topsoil a unique and indispensable part of the soil profile.
Addition of nitrogen. Bacteria living in nodules on legume roots convert nitrogen from atmospheric gas (N2) to forms that the plant can use directly. A number of free-living bacteria also fix nitrogen.
Storage of nutrients on soil organic matter. Decomposing organic matter can feed plants directly, but it also can indirectly benefit the nutrition of the plant. A number of essential nutrients occur in soils as positively charged molecules called cations (pronounced cat-eye-ons). The ability of organic matter to hold on to cations in a way that keeps them available to plants is known as cation exchange capacity (CEC). Humus has many negative charges, and because opposite charges attract, it is able to hold on to positively charged nutrients, such as calcium (Ca++), potassium (K+), and magnesium (Mg++) (see Figure 2.5a). This keeps them from leaching (washing through the soil) deep into the lower soil. Nutrients held in this way can be gradually released into the soil solution and made available to plants throughout the growing season. However, keep in mind that not all plant nutrients occur as cations. For example, the nitrate form of nitrogen is negatively charged (NO3–) and is actually repelled by the negatively charged CEC. Therefore, nitrate leaches easily as water moves down through the soil and beyond the root zone.
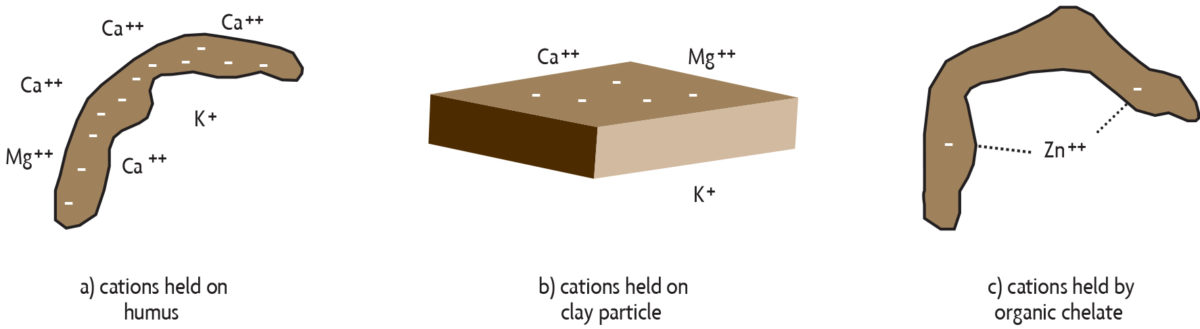
Clay particles also have negative charges on their surfaces (Figure 2.5b), but organic matter may be the major source of negative charges for coarse and medium-textured soils. Some types of clays, such as those found in the southeastern United States and in the tropics, tend to have low amounts of negative charge. When those clays are present, organic matter is even more critical as it is the main source of negative charges that bind nutrients.
Protection of nutrients by chelation. Organic molecules in the soil may also hold on to and protect certain nutrients. These particles, called chelates (pronounced key-lates) are byproducts of the active decomposition of organic materials or are secreted from plant roots. In general, elements are held more strongly by chelates than by binding of positive and negative charges. Chelates work well because they bind the nutrients at more than one location on the organic molecule (Figure 2.5c). In some soils, trace elements, such as iron, zinc and manganese, would be converted to unavailable forms if they were not bound by chelates. It is not uncommon to find low-organic-matter soils or exposed subsoils deficient in these micronutrients.
Other ways of maintaining available nutrients. There is some evidence that organic matter in the soil can inhibit the conversion of available phosphorus to forms that are unavailable to plants. One explanation is that organic matter coats the surfaces of minerals that can bond tightly to phosphorus. Once these surfaces are covered, available forms of phosphorus are less likely to react with them. In addition, some organic molecules may form chelates with aluminum and iron, both of which can react with phosphorus in the soil solution. When they are held as chelates, these metals are unable to form an insoluble mineral with phosphorus.
Beneficial Effects of Soil Organisms
Soil organisms are essential for keeping plants well supplied with nutrients because they break down organic matter, including other dead organisms. These organisms make nutrients available by freeing them from organic molecules. Some bacteria fix nitrogen gas from the atmosphere, making it available to plants. Other organisms dissolve minerals and make phosphorus more available. Without sufficient food sources, soil organisms aren’t plentiful and active, and consequently more fertilizers will be needed to supply plant nutrients.
ORGANIC MATTER INCREASES THE AVAILABILITY OF NUTRIENTS …
Directly
- As organic matter is decomposed, nutrients are converted into forms that plants can use directly.
- CEC is produced during the decomposition process, increasing the soil’s ability to retain calcium, potassium, magnesium and ammonium.
- Organic molecules are produced that hold and protect a number of micronutrients, such as zinc and iron.
- Some organisms make mineral forms of phosphorus more soluble while others fix nitrogen, which converts it into forms that other organisms or plants may use.
Indirectly
- Substances produced by microorganisms promote better root growth and healthier roots. With a larger and healthier root system, plants are able to take up nutrients more easily.
- Organic matter improves soil structure, which results in increased water infiltration following rains and increased water-holding capacity of the soil; it also enhances root growth into more permeable soil. This results in better plant health and allows more movement of mobile nutrients (such as nitrates) to the root.
A varied community of organisms is your best protection against major pest outbreaks and soil fertility problems. A soil rich in organic matter and continually supplied with different types of fresh residues, through the use of cover crops, complex rotations and applied organic materials such as compost or animal manure, is home to a much more diverse group of organisms than soil depleted of organic matter. The residues provide sufficient food sources to maintain high populations of soil organisms. There are two aspects to biological diversity, both aboveground and belowground: 1) the range of different organisms present and 2) their relative populations (referred to as evenness). It’s good to have diverse species of organisms, but it is a richer environment when there are also similar population sizes. For example, if there is a moderate population of disease organisms, we don't just want a small population of beneficial organisms present; the soil is biologically richer if there is also a moderate population of beneficials. Good populations of diverse organisms help ensure that fewer potentially harmful organisms will be able to develop sufficient numbers to reduce crop yields.
Soil Tilth
When soil has a favorable physical condition for growing plants, it is said to have good tilth. Such a soil is porous and allows water to enter easily, instead of running off the surface (Figure 2.6). More water is stored in the soil for plants to use between rains, and less erosion occurs. Good tilth also means that the soil is well aerated. Roots can easily obtain oxygen and get rid of carbon dioxide. A porous soil does not restrict root development and exploration. When a soil has poor tilth, its structure deteriorates and soil aggregates break down, causing increased compaction and decreased aeration and water storage. A soil layer can become so compacted that roots can’t grow. A soil with excellent physical properties will have numerous channels and pores of many different sizes.
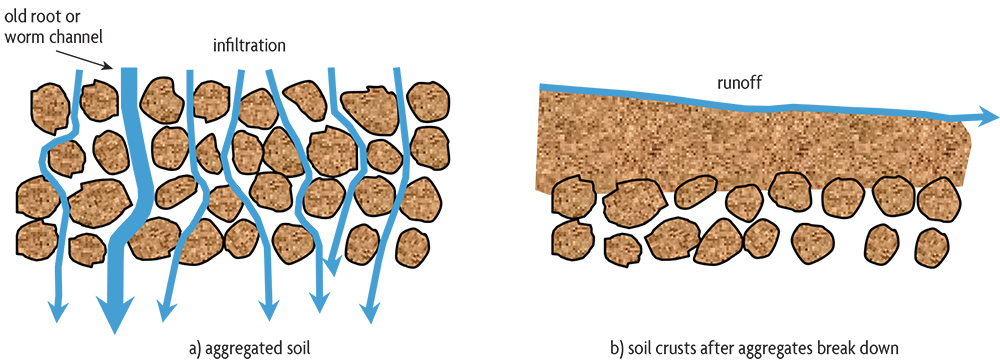
Studies on both undisturbed and agricultural soils show that as organic matter increases, soils tend to be less compact and have more space for air passage, helping to conduct water into the soil and storing it for plants to use. Sticky substances are produced during the decomposition of plant residues. Along with plant roots and fungal hyphae, they bind mineral particles together into clumps, or aggregates. In addition, the sticky secretions of mycorrhizal fungi—beneficial fungi that enter roots while growing thin filaments into the soil that help plants get more water and nutrients—are important binding material in soils. The arrangement and collection of individual particles as aggregates and the degree of soil compaction have huge effects on plant growth (see chapters 5 and 6). The development of aggregates is desirable in all types of soils because it promotes better drainage, aeration and water storage. The one exception is for some wetland crops, such as rice, where you want a dense soil that keeps fields flooded. (Although newer rice-growing systems show that high yields can be obtained with less flooding, thereby saving water.)
Organic matter, as residue on the soil surface or as a binding agent for aggregates near the surface, plays an important role in decreasing soil erosion. As with leaves and stems of living plants, surface residues intercept raindrops and decrease their potential to detach soil particles. These surface residues also slow water as it flows across the field, giving it a better chance to infiltrate into the soil. Aggregates and large channels greatly enhance the ability of soil to conduct water from the surface into the subsoil. Larger pores are formed in a number of ways. Old root channels may remain open for some time after the root decomposes. Larger soil organisms, such as insects and earthworms, create channels as they move through the soil. The mucus that earthworms secrete to keep their skin from drying out also helps to keep their channels open for a long time.
Most farmers can tell that one soil is better than another by looking at them, seeing how they work up when tilled, or even by sensing how they feel when walked on or touched. What they are seeing or sensing is really good tilth. And digging a bit into the soil can give a sense of its porosity and extent of aggregation.
Since erosion tends to remove the most fertile part of the soil, it can cause a significant reduction in crop yields. In some soils, the loss of just a few inches of topsoil may result in a yield reduction of 50%. The surface of some soils low in organic matter may seal over, or crust, as rainfall breaks down aggregates and as pores near the surface fill with solids. When this happens, water that can’t infiltrate into the soil runs off the field, carrying away valuable topsoil (Figure 2.6).
Protection of the Soil Against Rapid Changes in Acidity

Acids and bases are released as minerals dissolve and organisms go about their normal functions of decomposing organic materials or fixing nitrogen. Acids or bases are excreted by the roots of plants, and acids form in the soil from the use of nitrogen fertilizers. It is best for plants if the soil acidity status, referred to as pH, does not swing too wildly during the season. The pH scale is a way of expressing the amount of free hydrogen (H+) in the soil water, but in soils it is strongly related to the availability of plant nutrients and toxicity of certain elements like aluminum. It is a log scale, so a soil at pH 4 is very acidic and its solution is 10 times more acidic than a soil at pH 5. A soil at pH 7 is neutral: there is just as much base in the water as there is acid. Most crops do best when the soil is slightly acid and the pH is around 6 to 7, although there are acid-loving crops like blueberries. Essential nutrients are more available to plants in this pH range than when soils are either more acidic or more basic. Soil organic matter is able to slow down, or buffer, changes in pH by taking free hydrogen out of solution as acids are produced or by giving off hydrogen as bases are produced. (For discussion about management of acidic soils, see Chapter 20).
Stimulation of Root Development
Humic substances in soil may stimulate root growth and development by both increasing availability of micronutrients and by changing the expression of a number of genes (Figure 2.7). Microorganisms in soils produce numerous substances that stimulate plant growth. These include a variety of plant hormones and chelating agents. The stimulation by chelating substances (siderophores) is mainly due to making micronutrients more available to plants, which causes roots to grow longer and to have more branches. In addition, free-living nitrogen fixing bacteria provide the plant with additional sources of that essential nutrient while some bacteria help dissolve phosphorus from minerals, which makes it more available to plants.
Darkening of the Soil
Organic matter tends to darken soils. You can easily see this in coarse-textured sandy soils containing light-colored quartz minerals. Under well-drained conditions, a darker soil surface allows a soil to warm up a little faster in the spring. This provides a slight advantage for seed germination and the early stages of seedling development, which is often beneficial in cold regions.
Protection Against Harmful Chemicals
Some naturally occurring chemicals in soils can harm plants. For example, aluminum is an important part of many soil minerals and, as such, poses no threat to plants. As soils become more acidic, especially at pH levels below 5.5, aluminum becomes soluble. Some soluble forms of aluminum, if present in the soil solution, are toxic to plant roots. However, in the presence of significant quantities of soil organic matter, the aluminum is bound tightly and will not do as much damage.
Organic matter is the single most important soil property that reduces pesticide leaching. It holds tightly on to a number of pesticides. This prevents or reduces leaching of these chemicals into groundwater and allows time for detoxification by microbes. Microorganisms can change the chemical structure of some pesticides, industrial oils, many petroleum products (gas and oils), and other potentially toxic chemicals, rendering them harmless.
Organic Matter And Natural Cycles
The Carbon Cycle
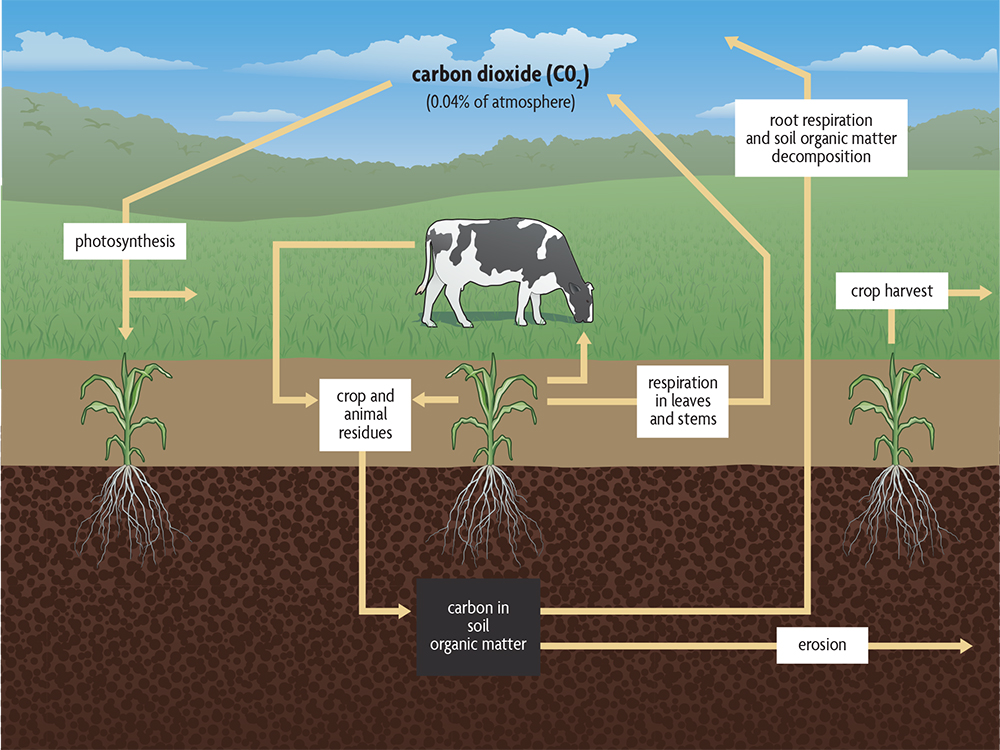
Soil organic matter plays a significant role in a number of global cycles. People have become more interested in the carbon cycle because the buildup of carbon dioxide in the atmosphere is the primary cause of climate destabilization.
A simple version of the natural carbon cycle that leaves out industrial sources, showing the role of soil organic matter, is given in Figure 2.8. Carbon dioxide is removed from the atmosphere by plants and used to make all the organic molecules necessary for life. Sunlight provides plants with the energy they need to carry out this process. Plants, as well as the animals feeding on plants, release carbon dioxide back into the atmosphere as they use organic molecules for energy. Carbon dioxide is also released to the atmosphere when fuels, such as gas, oil, coal and wood are burned.
COLOR AND ORGANIC MATTER
In Illinois, a handheld chart has been developed to allow people to estimate percent of soil organic matter. Their darkest soils, almost black, indicate 3.5–7% organic matter. A dark brown soil indicates 2–3%, and a yellowish brown soil indicates 1.5–2.5% organic matter. (Color may not be as clearly related to organic matter in all regions because the amount of clay and the types of minerals also influence soil color.) Recently, mobile apps have been developed that use smartphone cameras to estimate soil organic matter content and have proven to work quite well for rough estimates.
Soils are amassing the cumulative carbon and nutrient capture from plant production, and the largest amount of carbon present on the land is not in the living plants but is instead stored in soil organic matter. It has taken a while, but that understanding is now finding its way into discussions of the carbon cycle. More carbon is stored in soils than in all plants, all animals and the atmosphere combined. Soil organic matter contains an estimated four times as much carbon as living plants, and in fact carbon stored in all the world’s soils is two to three times the amount in the atmosphere. As soil organic matter is depleted, it becomes a source of carbon dioxide for the atmosphere. Also, when forests are cleared and burned, a large amount of carbon dioxide is released. A secondary, often larger flush of carbon dioxide is emitted from soil through the rapid depletion of soil organic matter following conversion of forests to agricultural practices. There is as much carbon in seven inches of a soil with 1% organic matter as there is in the atmosphere above a field. If organic matter decreases from 3% to 2%, the amount of carbon dioxide in the atmosphere could double. (Of course, wind and diffusion move the carbon dioxide to other parts of the globe, and it can be absorbed by the oceans and taken up by plants downwind during photosynthesis.)
Climate Change and Soils
Climate change is already having profound effects on the planet by warming seas, melting glaciers and sea ice, thawing frozen soil (permafrost), and increasing weather extremes: more heat waves, increasing intensity of rainfall in many places and more frequent dry conditions in other locations. As we write this, the last five years (2015, 2016, 2017, 2018 and 2019) have been the warmest since record keeping began in the 1880s. The 2018 and 2019 heat waves in North America, Europe, and southeast and eastern Asia, as well as during the following Australian summer (beginning in December 2018 and then again in their 2019–2020 summer, accompanied this time by historic wildfires), have been especially severe. July 2019 was the warmest month ever recorded. Farming has already been affected in many parts of the world, with increasing night temperatures lowering grain yields as more energy that plants produce during the day is used up by greater nighttime respiration, and with regional droughts causing crop failures.Gases such as carbon dioxide (CO2), methane (CH3) and nitrous oxide (N2O) trap heat in the atmosphere, resulting in a warming Earth, the so-called greenhouse effect.
Atmospheric carbon dioxide concentrations increased from around 320 parts per million (ppm) in the mid 1960s to 415 ppm as we write these words, and it is increasing at the rate of about 2 to 3 ppm per year. The historical conversion of forests and grasslands to farming was responsible for a large transfer of carbon (from accelerated soil organic matter decomposition) into the atmosphere as CO2. This agricultural conversion is second to the burning of fossil fuels as the largest contributor to increasing atmospheric CO2 concentrations (remember, fossil fuels are derived from carbon stored in ancient plants). As forests are burned and soils are plowed in order to grow crops (enhancing the use of organic matter by soil organisms), CO2 is emitted into the atmosphere.
But soils managed in ways that build up organic matter can become net sinks for carbon storage and can enhance their health at the same time. Increasing soil organic matter is no silver bullet for combating climate change, but it can help to slow the increase in CO2 for a while if done on a massive scale all over the world. A number of non-governmental organizations in the United States, along with a number of international efforts, are encouraging farmers to increase soil organic matter levels in the form of payments for sequestering carbon. (Large-scale “geoengineering” schemes have been proposed to take CO2 out of the atmosphere or to shoot particles into the atmosphere to reflect some of the incoming radiation from the sun. The costs and potentially negative side effects of such proposals have not been established. Thus, at present, drastically reducing fossil fuel use through switching to renewable energy sources and reducing total energy use is the only sure way we know to stop or reverse climate change.)Ecologically sound management of agricultural soils using practices that promote the buildup of organic matter certainly has a part to play in combating climate change. It offers win-win outcomes because higher levels of organic matter also increase resilience of soils that are being confronted with the more intense storms and dry periods resulting from a warming planet with increasingly destabilized weather patterns. Read further about the role of soil health in climate resilience in the SARE bulletin Cultivating Climate Resilience on Farms and Ranches (www.sare.org/climate-resilience).
The Nitrogen Cycle
Gains. Another important global process in which organic matter plays a major role is the nitrogen cycle. It is of direct importance in agriculture because there is frequently not enough available nitrogen in soils for plants to grow their best. Both nitrate and ammonium can be used by plants, but most nitrogen used by plants is taken up in the nitrate form, with a small amount as ammonium. Small quantities of some sources of amino acids and small proteins can be absorbed. Figure 2.9 shows the nitrogen cycle and how soil organic matter enters into the cycle. Almost all of the nitrogen in soils exists as part of the organic matter, in forms that plants are not able to use as their main nitrogen source. Every percent organic matter in a surface soil (to 6 inches deep) contains approximately 1,000 pounds of nitrogen. Each year bacteria and fungi convert some portion of the organic forms of nitrogen into ammonium, and different bacteria convert ammonium into nitrate. Depending on the soil organic matter levels, a typical crop may derive 20–50% of its nitrogen from mineralized organic matter.
Animal manures can also make large contributions to the plant-available nitrogen pool in the soil. They typically have high organic nitrogen contents that are made readily available when microorganisms convert organic forms to ammonium and nitrate. Most of the crop’s nitrogen demand can be met with manure on livestock farms where large amounts of it are generated.
In addition to decomposing organic matter and manure, nitrogen is also derived from some bacteria living in soils that can “fix” nitrogen, converting nitrogen gas to forms that other organisms, including crop plants, can use. These can be modest amounts of nitrogen in typical cereal crop systems but large quantities when growing a legume. Also, inorganic forms of nitrogen, like ammonium and nitrate, exist in the atmosphere naturally and are sometimes enhanced by air pollution. Rainfall and snow deposit these inorganic nitrogen forms on the soil, but generally in modest amounts relative to the needs of a typical crop. Inorganic nitrogen also may be added in the form of commercial nitrogen fertilizers, which for most cash grain crops (except legumes like soybeans) is generally the largest nitrogen addition. These fertilizers are derived from nitrogen gas in the atmosphere through an industrial fixation process that requires quite a lot of energy.
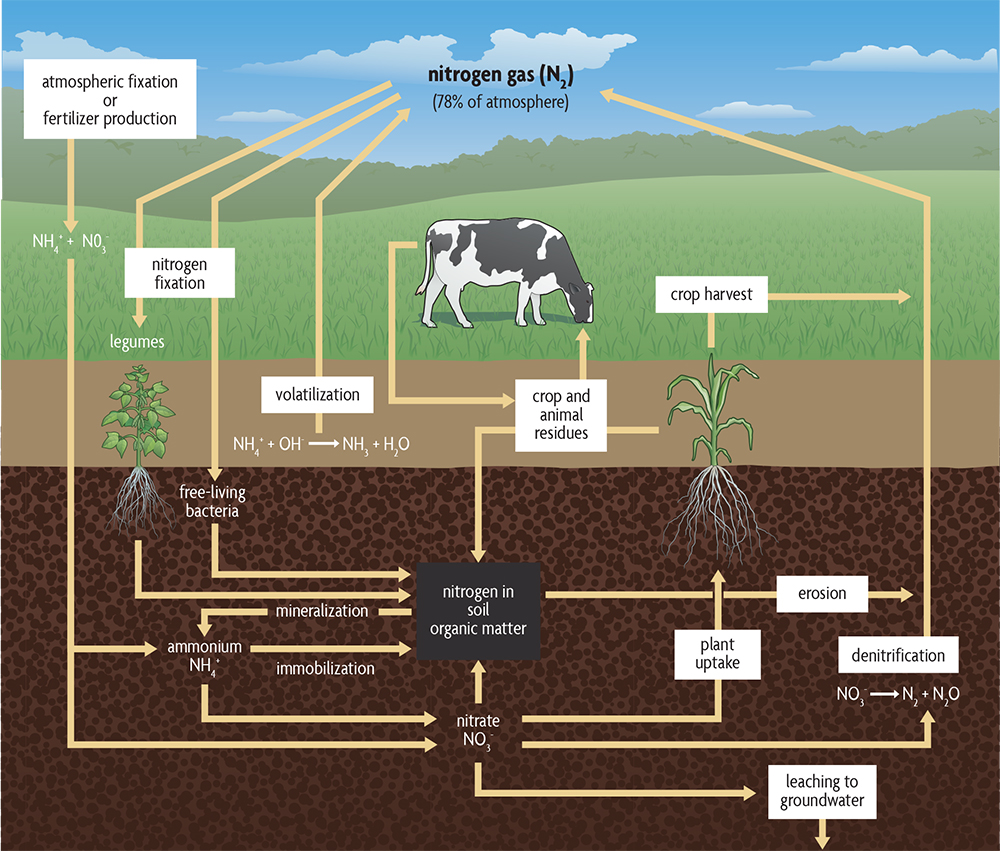
Losses. Nitrogen can be lost from a soil in a number of ways. Soil conditions and agricultural practices govern the extent of loss and the way in which nitrogen is lost. When crops are removed from fields, nitrogen and other nutrients also are removed. When uncomposted manure or certain forms of nitrogen fertilizer are placed on the soil surface, gaseous losses (volatilization) may occur, which may cause losses of up to 30%. The nitrate (NO3–) form of nitrogen leaches readily from soils and may end up in groundwater at levels unsafe for drinking or may enter surface waters where it causes low-oxygen “dead zones.” Leaching losses are greatest in sandy soils and in soils with tile drainage. Organic forms of nitrate, as well as nitrate and ammonium (NH4+), may be lost by runoff water and erosion.Once freed from soil organic matter, nitrogen may be converted to forms that end up back in the atmosphere. Bacteria convert nitrate to nitrogen (N2) and to nitrous oxide (N2O) gases in a process called denitrification, which can be a significant pathway of loss from soils that are saturated. Nitrous oxide (also a potent greenhouse gas) contributes strongly to climate change, and in fact is estimated to be the largest agricultural contribution to greenhouse gas emissions (more than carbon dioxide and methane). In addition, when it reaches the upper atmosphere, it decreases ozone levels that protect the earth’s surface from the harmful effects of ultraviolet (UV) radiation. So if you needed another reason to use nitrogen fertilizers and manures efficiently—in addition to the economic costs and the pollution of ground and surface waters—the possible formation of nitrous oxide should make you cautious.
The Water Cycle
Organic matter plays an important part in local, regional and global water cycles due to its role in promoting water infiltration into soils and storage within the soil. The water cycle is also referred to as the hydrologic cycle. Water evaporates from the soil surface and from living plant leaves as well as from oceans and lakes. Water then returns to the earth, usually far from where it evaporated, as rain and snow. Soils high in organic matter, with excellent tilth, enhance the rapid infiltration of rainwater into the soil and increase storage of water in soil. When we look at the increasing occurrence of major flooding in parts of the world, especially in the U.S. grain belt, we point to climate change. But surely this is worsened by the gradual degradation of regional soils that are mostly used for intensive crop production.
The water that has entered the soil may be available for plants to use or it may percolate deep into the subsoil and help to recharge the groundwater supply. Since groundwater is commonly used as a drinking water source for homes and for irrigation, recharging groundwater is important. When the soil’s organic matter level is depleted, it is less able to accept and store water, and high levels of runoff and erosion result. This means less water for plants and decreased groundwater recharge.
VALUE OF SOIL ORGANIC MATTER
It is very difficult, if not impossible, to come up with a meaningful monetary value for the worth of organic matter in our soils. It positively affects so many different properties that taking them all into account and figuring out their dollar value is an enormous task. One percent organic matter in the top 6 inches of an acre of soil contains about 1,000 pounds of nitrogen. At about 45 cents per pound, this alone is worth about $450 for every percent organic matter in your soil. Adding in the value of 100 pounds each of phosphorus, sulfur and potassium, the total comes to $500 per acre for every percent of organic matter. But we also need to consider other nutrients that are present and the beneficial effects that organic matter has on reducing other inputs and increasing yields. And what are the monetary benefits of reduced flooding, water pollution and climate change? We know it truly is an invaluable resource, but it is difficult to put an exact price on it.
Chapter 2 Summary
Soil organic matter is the key to building and maintaining healthy soils because it has such great positive influences on essentially all soil properties—aggregation, nutrient availability, soil tilth and water availability, biological diversity and so on— helping to grow healthier plants. Organic matter consists mainly of the living organisms in the soil (“the living”), the fresh residue (“the dead”), and the well-decomposed (or burned) material physically or chemically protected from decomposition (“the very dead”). Residue trapped inside aggregates (a portion of “the dead” organic matter), especially small ones, is protected also from decomposition because organisms are unable to access the material. Each of these types of organic matter plays an important role in maintaining healthy soils. Soil organic matter transformations are a key part of plant nutrition and the ability to achieve good crop yields. Soil organic matter is also an integral part of local and global cycles of carbon, nitrogen and water, impacting many aspects that define the sustainability and future survival of life on earth.
Chapter 2 Sources
Allison, F.E. 1973. Soil Organic Matter and Its Role in Crop Production. Scientific Publishing Co.: Amsterdam, Netherlands.
Brady, N.C. and R.R. Weil. 2008. The Nature and Properties of Soils, 14th ed. Prentice Hall: Upper Saddle River, NJ.
Follett, R.F., J.W.B. Stewart and C.V. Cole, eds. 1987. Soil Fertility and Organic Matter as Critical Components of Production Systems. Special Publication No. 19. Soil Science Society of America: Madison, WI.
Lal, R. 2008. Sequestration of atmospheric CO2 in global carbon pools. Energy & Environmental Science 1.
Lehmann, J., D.C. Kern, B. Glaser and W.I. Woods, eds. 2003. Amazonian Dark Earths: Origin, Properties, Management. Kluwer Academic Publishing: Dordrecht, Netherlands.
Lehmann, J. and M. Rondon. 2006. Bio-char soil management on highly weathered soils in the humid tropics. In Biological Approaches to Sustainable Soil Systems, ed. N. Uphoff et al., pp. 517–530. CRC Press: Boca Raton, FL.
Lucas, R.E., J.B. Holtman and J.L. Connor. 1977. Soil carbon dynamics and cropping practices. In Agriculture and Energy, ed. W. Lockeretz, pp. 333–451. Academic Press: New York, NY. (See this source for the Michigan study on the relationship between soil organic matter levels and crop-yield potential.)
Manlay, R.J., C. Feller and M.J. Swift. 2007. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agriculture, Ecosystems and Environment 119: 217–233.
Oliveira Nunes, R., G. Abrahão Domiciano, W. Sousa Alves, A. Claudia A. Melo, Fábio Cesar, S. Nogueira, L. Pasqualoto Canellas and F. Lopes Olivares. 2019. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Scientific Reports (NatureReports) 9, Article number: 12019. Accessed Sept. 14, 2019, at https://www.nature.com/articles/s41598-019-48509-2.
Oshins, C. and L. Drinkwater. 1999. An Introduction to Soil Health. An unpublished slide set.
Powers, R.F. and K. Van Cleve. 1991. Long-term ecological research in temperate and boreal forest ecosystems. Agronomy Journal 83: 11–24. (This reference compares the relative amounts of carbon in soils with that in plants.)
Stevenson, F.J. 1986. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients. John Wiley & Sons: New York, NY. (This reference compares the amount of carbon in soils with that in plants.)
Strickling, E. 1975. Crop sequences and tillage in efficient crop production. Abstracts of the 1975 Northeast Branch American Society Agronomy Meetings: 20–29. (See this source for the Maryland experiment relating soil organic matter to corn yield.)
Tate, R.L., III. 1987. Soil Organic Matter: Biological and Ecological Effects. John Wiley & Sons: New York, NY.
U.S. Environmental Protection Agency. 2019. Inventory of U.S. greenhouse gas emissions and sinks. EPA 430-R-19-001. Available at www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks.Weil, R. and F. Magdoff. 2004. Significance of soil organic matter to soil quality and health. In Soil Organic Matter in Sustainable Agriculture, ed. F. Magdoff and R. Weil, pp. 1–43. CRC Press: Boca Raton, FL.
