The purchase of plant food is an important matter, but the use of a [fertilizer] is not a cure-all, nor will it prove an adequate substitute for proper soil handling.
—J.L. Hills, C.H. Jones and C. Cutler, 1908
Most of the essential nutrients for plants, animals and humans are derived from weathered minerals in the soil. But plants also absorb carbon, oxygen and hydrogen from the air and water. Nitrogen is derived from the atmosphere by legumes, but other plants absorb it from the soil. Of the 17 elements needed by all plants (Table 18.1), only three—nitrogen (N), phosphorus (P) and potassium (K)—are commonly deficient in soils. Deficiencies of sulfur (S) are less prevalent but not uncommon. Other nutrients, such as magnesium (Mg), zinc (Zn), boron (B) and manganese (Mn), can be lacking in certain regions. Deficiencies of sulfur, magnesium and some micronutrients may be more common in regions with highly weathered minerals, such as the southeastern United States and many parts of the tropics, or those with high rainfall, such as portions of the Pacific Northwest. Sulfur deficiency is especially common on the sandy soils on the coastal plains of the Southeast and has become more common in areas with low organic matter soils with the decrease in sulfur air pollution from coal burning power plants. Keep an eye out for deficiencies of iron, zinc, copper and manganese on higher-pH calcareous soil, especially in drier regions. Low phosphorus availability is also common in calcareous soils. In contrast, in locations with relatively young soil that contains minerals that haven’t been extensively weathered by nature, such as glaciated areas with moderate to low rainfall like the Dakotas, K deficiencies are less common.
Environmental concerns have resulted in more emphasis on better management of N and P over the past few decades. While these nutrients are critical to soil fertility management, their mismanagement also causes widespread environmental problems. In many regions of the United States and other countries, surface and groundwater pollution has been caused by poor soil management, overuse of fertilizers, mishandling of manures, sewage sludges (biosolids) and composts, and high animal numbers on limited land areas. Because N and P are used in large quantities and their overuse has potential environmental implications, we’ll discuss them together in Chapter 19. Other nutrients, cation exchange, soil acidity (low pH) and liming, and arid and semiarid region problems with sodium, alkalinity (high pH), and excess salts are covered in Chapter 20.
The Bottom Line: Nutrients and Plant Health, Pests, Profits, and the Environment
Management practices are all related. The key is to visualize them all as part of whole-farm management, leading you to the goals of better crop growth and better quality. Plants should be healthy and have large root systems if a soil has good tilth, no subsurface compaction, good drainage, adequate water, a good supply of organic matter and a thriving soil biological community. This enables plants to efficiently take up nutrients and water from the soil and to use those nutrients to produce higher yields. Higher yields also imply indirect benefits like more carbon capture from the atmosphere and better water cycling.
Doing a good job of managing nutrients on the farm and in individual fields is critical to general plant health and management of plant pests. Too much available N in the early part of the growing season allows small-seeded weeds, with few nutrient reserves, to get well established. This early jump-start may then enable them to out-compete crop plants later on. Restricted plant growth may occur if nutrients aren’t present at the right time of the season in sufficient quantities and in reasonable balance to one another. Plants under nutrient stress may be stunted if nutrient levels are low, or they may grow too much foliage and not enough fruit if N is too plentiful relative to other nutrients. Plants under nutrient stress grow abnormally, for example, in the presence of too low or too high N levels, and are not able to emit as much of the natural chemicals that signal beneficial insects capable of fighting insect pests that feed on leaves or fruit. Low K levels aggravate stalk rot of corn and winter damage to bermudagrass. On the other hand, pod rot of peanuts is associated with excess K within the fruiting zone of peanuts (the top 2–3 inches of soil). Blossom-end rot of tomatoes is related to low calcium levels, often made worse by droughty conditions, or irregular rainfall or poor irrigation.
Economic returns will be reduced when plants don’t grow well. Yield and crop quality usually are lower, reducing the amount of money received. There also may be added costs to control pests that take advantage of crops with poor nutrient management. In addition, when nutrients are applied beyond plant needs, it’s like throwing money away. Entire communities may suffer from poor water quality when N and P are lost from the soil by leaching to groundwater or running into surface water.
THE 4Rs OF NUTRIENT STEWARDSHIP
The risks of high environmental impacts and lower crop yields are reduced when fertilizer materials are properly managed. The concept of 4R nutrient stewardship is a set of principles for good nutrient management (maximizing nutrient-use efficiency and minimizing environmental impacts) that recognizes that the best practices vary by local soil, climate and management factors. The 4Rs encapsulate the practices that we discuss in this chapter:
- Right fertilizer source at the
- Right rate, at the
- Right time, and in the
- Right place
Taking this concept even further, 4R-Plus combines the 4R management practices with conservation practices that enhance soil health and improve the environment. 4R and 4R-Plus are therefore useful concepts that summarize some of the multi-faceted concepts we discuss in this book.
Organic Matter and Nutrient Availability
The best overall strategy for nutrient management is to enhance the level of organic matter in soils (Figure 18.1). This is especially true for N and P. Soil organic matter, together with any freshly applied residues, are well-known sources of N for plants. (However, as discussed in Chapter 9, unusual residues with high C:N ratios can reduce N availability for a period of time.)
Mineralization of P and sulfur from organic matter is an important source of these nutrients. Also, organic matter helps hold on to positively charged potassium (K+), calcium (Ca++) and magnesium (Mg++) ions, and provides natural chelates that maintain micronutrients such as zinc, copper and manganese in forms that plants can use. In addition, the improved soil structure (tilth) and the growth-promoting substances produced during organic matter decomposition help the plant develop a more extensive root system, allowing it to obtain nutrients from a larger volume of soil. And a wide diversity of soil organisms helps maintain low populations of plant pathogens.
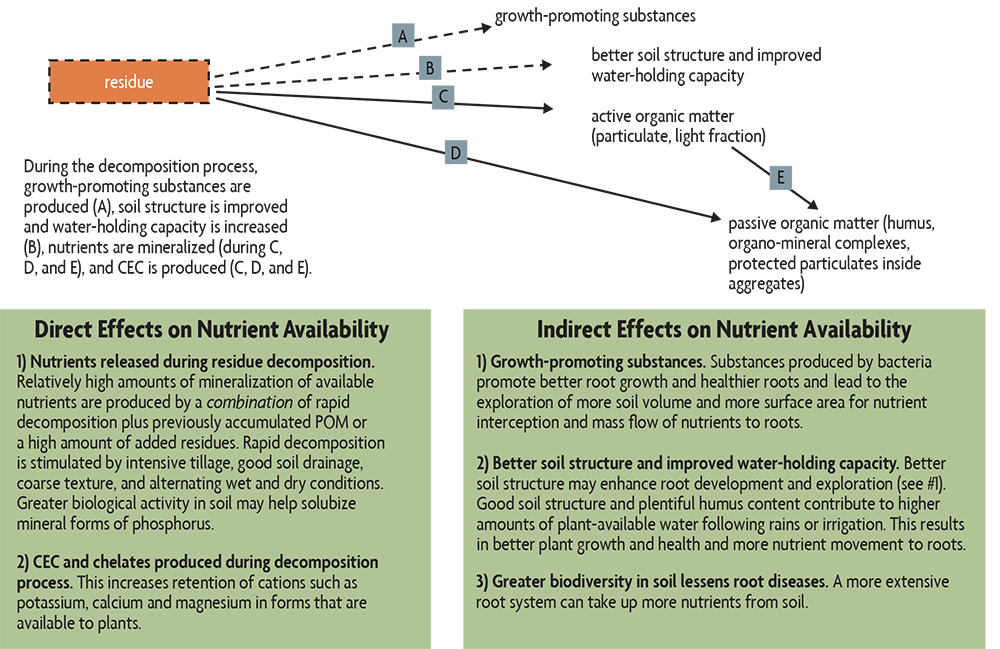
Cover crop roots (living soil organic matter) also contribute to nutrient management. They provide energy material that allows soil organisms to better thrive and mobilize soil nutrients, keep nutrients from being lost by leaching or runoff, add new N to the soil (if a legume), and maintain plentiful supplies of mycorrhizae spores that lead to better inoculation of the following crop, helping it to take up soil nutrients.
Improving Nutrient Cycling on the Farm
For economic and environmental reasons, it makes sense for plants to more efficiently utilize nutrient cycling on the farm. Goals should include a reduction in long-distance nutrient flows, as well as the promotion of “true” on-farm cycling, in which nutrients return in the form of crop residue or manure to the fields from which they came. There are a number of strategies to help farmers reach the goal of better nutrient cycling:
- Reduce unintended losses by promoting water infiltration and better root health through enhanced management of soil organic matter and physical properties. Methods to increase and improve organic matter status include additions of a variety of sources of organic materials as well as methods for reducing losses from tillage and adopting conservation practices. Proper irrigation water management involves applying the right amount of irrigation water needed to refill the root zone. Applying excessive irrigation water can cause both runoff and leaching losses of nutrients. (In arid climates occasional extra water applications will be needed to leach accumulating salts below the root zone.) In addition, compared to conventional annual row crops such as corn and soybeans, rotations that include cover crops and perennial grass and legume crops tend to result in less leaching loss of nitrate as well as runoff phosphorus loss.
THE ABCs OF NUTRIENT MANAGEMENT
a. Balance nutrient inflows and removals to maintain optimal levels and allow a little “drawdown” if nutrient levels get too high.
b. Enhance soil structure to increase plant capture of soil nutrients and reduce their loss in runoff by minimizing tillage, reducing compaction and promoting deeper rooting to access nutrients lower in the soil.
c. Build up and maintain high soil organic matter levels for biodiverse soils and to develop healthy plant roots.
d. Test manures and credit their nutrient content before applying fertilizers or other amendments.
e. If using liquid manure, consider soil injection to reduce N volatilization and potential loss of nutrients in runoff.
f. Test soils regularly to determine the nutrient status and whether or not manures, fertilizers or lime are needed.
g. Use regionally adapted nutrient recommendation tools.
h. Apply most nitrogen close to the time of crop uptake, and use recommendation tools that account for soil, weather and management practices.
i. Use forage legumes or legume cover crops to provide N to subsequent crops and to develop good soil structure.
j. Use cover crops to tie up nutrients during the off-season, enhance soil structure, reduce runoff and erosion, and provide microbes with fresh organic matter.
k. Maintain soil pH in the optimal range for the most sensitive crops in your rotation.
l. When P and K are very deficient, broadcast some of the fertilizer to increase the general soil fertility level, and band apply some as well.
m. To get the most efficient use of a fertilizer when P and K levels are at or below the medium or lower categories, consider band application at planting, especially in cool climates.
| Table 18.1 Essential Nutrients for Plants | ||
|---|---|---|
| Element | Common available form | Source |
| Needed in large amounts | ||
| Carbon | CO2 | atmosphere |
| Oxygen | O2, H2O | atmosphere and soil pores |
| Hydrogen | H2O | water in soil pores |
| Nitrogen | NO3–, NH4+ | soil (atmosphere for legumes) |
| Phosphorus | H2PO4–, HPO4–2 | soil |
| Potassium | K+ | soil |
| Calcium | Ca+2 | soil |
| Magnesium | Mg+2 | soil |
| Sulfur | SO4–2 | soil |
| Needed in small amounts | ||
| Iron | Fe+2, Fe+3 | soil |
| Manganese | Mn+2 | soil |
| Copper | Cu+, Cu+2 | soil |
| Zinc | Zn+2 | soil |
| Boron | H3BO3 | soil |
| Molybdenum | MoO4–2 | soil |
| Chlorine | Cl– | soil |
| Nickel | Ni+2 | soil |
| Needed by some plants1, 2 | ||
| Cobalt | Co+2 | soil |
| Sodium | Na+ | soil |
| Silicon | H4SiO4 and H2SiO4–2 | soil |
| 1Cobalt has been shown to be essential only for legumes; sodium (Na) is considered an essential element for some plants; and silicon (Si) is considered essential for the normal growth and health of rice. 2Although selenium (Se) is not considered an essential element for plants, it is essential for animals, and so the Se content of plants is important for animal nutrition. On the other hand, plants growing on high-Se soils (such as locoweed, asters and saltbushes) accumulate enough Se to become toxic to grazing animals. | ||
- Enhance nutrient uptake efficiency by carefully using fertilizers and amendments, as well as irrigation practices. Better placing and synchronizing nutrient applications with plant growth improve efficiency of fertilizer nutrients. Sometimes changing planting dates or switching to a new crop creates a better match between the timing of nutrient availability and crop needs.
- Tap local nutrient sources by seeking local organic materials, such as leaves or grass clippings from towns, aquatic weeds harvested from lakes, produce waste from markets and restaurants, food processing wastes and clean sewage sludges (see discussion on sewage sludge in Chapter 9). Caution always makes sense when receiving organic materials from off the farm; for example, grass might have been treated with herbicide, and municipal leaves may contain extraneous materials. Although some of these do not contribute to true nutrient cycles, the removal of agriculturally usable nutrients from the “waste stream” makes sense and helps develop more environmentally sound nutrient flows. The Food Safety Modernization Act (FSMA) requires greater care with use of certain organic materials, such as manures, when growing produce for the fresh market due to the potential for food to be contaminated with pathogens. Composting the materials from on or off the farm may be needed to comply with these regulations.
- Promote consumption of locally produced foods by supporting local markets as well as by returning local food wastes to farmland. When people purchase locally produced foods, there are more opportunities for true nutrient cycling to occur. Some community supported agriculture (CSA) farms, where subscriptions for produce are paid before the start of the growing season, encourage their members to return produce waste to the farm for composting, and a portion of the nutrients in the produce complete a true cycle.
- Reduce exports of nutrients in farm products by adding animal enterprises to crop farms. The best way to reduce nutrient exports per acre, as well as to make more use of forage legumes in rotations, is to add an animal (especially a ruminant) enterprise to a crop farm. Compared with selling crops, feeding crops to animals and exporting animal products result in far fewer nutrients and carbon leaving the farm. Keep in mind that, on the other hand, raising animals with mainly purchased feed overloads a farm with nutrients.
- Bring animal densities in line with the land base of the farm. Renting or purchasing more land—to grow a higher percentage of animal feeds and to have increased area for manure application—or limiting animal numbers are ways to accomplish this.
- Develop local partnerships to balance flows among different types of farms. As pointed out in Chapter 9 when we discussed organic matter management, sometimes neighboring farmers cooperate with both nutrient management and crop rotations. This is especially beneficial when a livestock farmer has too many animals and imports a high percentage of feed, and a neighboring vegetable or grain farmer has a need for nutrients and an inadequate land base for allowing a rotation that includes a forage legume. Both farms win by cooperating on nutrient management and rotations, sometimes in ways that were not anticipated (see “Win-Win Cooperation” box), but it is more of a challenge as the distances become greater. As of January 2020, the Food Safety Modernization Act requires a range of practices and documentation for all farms selling more than $25,000 worth of products. The implications of this legislation for farm practices is discussed in Chapter 12, on integrating livestock and cropping.
NUTRIENT MANAGEMENT GOALS
- Satisfy crop nutrient requirements for optimum economic yield and quality.
- Minimize pest pressure caused by excess N fertilizer (such as from sap-feeding insects) or by a nutrient deficiency (low K causes less wheat resistance to rust and corn to stem rot).
- Minimize the environmental and economic costs of supplying nutrients.
- Use local sources of nutrients whenever possible.
- Get full nutrient value from fertility sources.
—Modified from OMAFRA, 1997
STRATEGIES FOR IMPROVING NUTRIENT CYCLES
- Reduce unintended losses.
- Enhance nutrient uptake efficiency.
- Tap local nutrient sources.
- Promote consumption of locally produced foods.
- Reduce off-farm exports of nutrients and carbon in farm products.
- Bring animal densities in line with the land base of the farm.
- Develop local partnerships to balance flows among different types of farms.
Some livestock farms that are overloaded with nutrients would like to transfer manure to other farms but find that transportation costs are a factor (manures contain up to 90% water). Separating liquids (which are high in N) from solids using a settling or mechanical screw press system can be helpful. Also, farmers are finding that composting is an attractive alternative way to handle manure. During the composting process, volume and weight are greatly reduced (see Chapter 13), resulting in less material to transport. Organic farmers are always on the lookout for reasonably priced animal manures and composts. The landscaping industry also uses a fair amount of compost. Local or regional compost exchanges can help remove nutrients from overburdened animal operations and place them on nutrient-deficient soils.
WIN-WIN COOPERATION
Cooperation between Maine potato farmers and their dairy farm neighbors has led to better soil and crop quality for both types of farms. As potato farmer John Dorman explains, after cooperating with a dairy farm on rotations and manure management, soil health “has really changed more in a few years than I’d have thought possible.” Dairy farmer Bob Fogler feels that the cooperation with the potato farmer allowed his family to expand the dairy herd. He notes, “We see fewer pests and better-quality corn. Our forage quality has improved. It’s hard to put a value on it, but forage quality means more milk.” —From Hoard’s Dairyman, April 10, 1999
Using Fertilizers and Amendments
There are four main questions to ask when applying nutrients:
- How much is needed?
- What source(s) should be used?
- When should the fertilizer or amendment be applied?
- How should the fertilizer or amendment be applied?
Chapter 21 details the use of soil tests to help you decide how much fertilizer or organic nutrient sources to apply. Here we will go over how to approach the other three issues.
Nutrient Sources: Commercial Fertilizers Versus Organic Materials
Numerous fertilizers and amendments are normally used in agriculture (some are listed in Table 18.1). Fertilizers such as urea, triple superphosphate and muriate of potash (potassium chloride) are convenient to store and use. They are also easy to blend to meet nutrient needs in specific fields and provide predictable effects. Their behavior in soils and the ready availability of the nutrients are well established. The timing, rate and uniformity of nutrient application are easy to control when using commercial fertilizers. However, there also are drawbacks to using commercial fertilizers. All of the commonly used N materials (those containing urea, ammonia and ammonium) are acid forming, and their use in humid regions, where native lime has been weathered and leached out, requires more frequent lime additions. The production of nitrogen fertilizers is also very energy intensive; it is estimated that, aside from solar energy that the crop uses, N fertilizers account for 25%–50% of the energy that goes into growing a corn crop. In addition, the high nutrient solubility can result in salt or ammonia damage to seedlings when excess fertilizer is applied close to seeds or plants. Nutrients in commercial fertilizers are readily available and allow for more precise timing with crop uptake, but if managed improperly they may become more readily lost to the environment compared to organic nutrient sources. (On the other hand, high rainfall events on a field with recently plowed-down alfalfa or applied manure may also result in significant nitrate leaching below the root zone.) Slow-release forms of synthetic nitrogen fertilizers such as sulfur- or polymer-coated urea help better match N availability to crop needs. Similarly, adding nitrification and urease inhibitors can facilitate more efficient nitrogen fertilizer use. Organic fertilizers generally contain a significant slow-release portion of N but are of such variable composition that it is difficult to know how much will be released in a given time. Feather meal, a commercially available processed organic fertilizer, is about 12%–13% nitrogen, with most released slowly.
Soils overloaded with either inorganic or organic sources of nutrients can be large sources of pollution. The key to wisely using either commercial fertilizers or organic sources is following recommendations based on soil tests, not applying more nutrients than the crop can use, and applying in ways and at times that minimize losses to the environment. Once the soil nutrient status is optimal, try to balance farm nutrient inflows and outflows. When nutrient levels, especially P, are in the high or very high range, stop application and try to maintain or “draw down” soil test levels. It usually takes years of cropping without adding P to lower soil test P appreciably. With grazing animals it can take a very long time because so few nutrients are being exported from the field and farm in animal products. On the other hand, when hay is harvested and sold off the farm, P drawdown can happen more rapidly.
| Table 18.2 Composition of Various Common Amendments and Commercial Fertilizers (%) | |||||||
|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | Ca | Mg | S | Cl | |
| N materials | |||||||
| Anhydrous ammonia | 82 | ||||||
| Aqua ammonia | 20 | ||||||
| Ammonium nitrate | 34 | ||||||
| Ammonium sulfate | 21 | 24 | |||||
| Calcium nitrate | 16 | 19 | 1 | ||||
| Urea | 46 | ||||||
| UAN solutions (urea + ammonium nitrate) | 28–32 | ||||||
| P and N+P materials | |||||||
| Superphosphate (ordinary) | 20 | 20 | 12 | ||||
| Triple superphosphate | 46 | 14 | 1 | ||||
| Diammonium phosphate (DAP) | 18 | 46 | |||||
| Monoammonium phosphate (MAP) | 11–13 | 48–52 | |||||
| K materials | |||||||
| Potassium chloride (muriate of potash) | 60 | 47 | |||||
| Potassium–magnesium sulfate (“K-Mag”) | 22 | 11 | 23 | 2 | |||
| Potassium sulfate | 50 | 1 | 18 | 2 | |||
| Other materials | |||||||
| Gypsum | 23 | 18 | |||||
| Limestone, calcitic | 25–40 | 0.5–3 | |||||
| Limestone, dolomitic | 19–22 | 6–13 | 1 | ||||
| Magnesium sulfate | 2 | 11 | 14 | ||||
| Potassium nitrate | 13 | 44 | |||||
| Sulfur (elemental S, gypsum, ammonium sulfate) | 30–99 | ||||||
| Wood ashes | 2 | 6 | 23 | 2 | |||
Are Organic Nutrient Sources Better for Soil and the Environment than Synthetic Fertilizers? The Answer is Complicated!
It is recommended to include organic nutrient sources as part of a nutrient management program to sustain soil health because they feed the plants while also better supporting soil biological functions. But on many farms commercial fertilizers are required to achieve good yields. Due to the structure of agriculture with associated nutrient flows (especially exports from grain production areas) and to the current inefficiencies in cropping systems, commercial fertilizers remain essential to feeding a growing global population. In fact, completely eliminating commercial fertilizers would not only cause a breakdown of the global food system, it would also negatively affect soil health. Inadequate nutrition of crops would reduce carbon capture from the atmosphere, biomass production and yields, and thereby also fresh carbon and nutrient supplies for the soil. Additional nutrients are critical to building organic matter in depleted soils (every ton of new carbon stored in the soil requires about 200 pounds of additional nitrogen and 30 pounds of phosphorus). Therefore, although organic matter is critical to building soil health, commercial fertilizers may be needed to achieve our goals.
At the global scale, commercial fertilizers are still critical to meeting the demands of our growing population until better practices (cover crops, better rotations, decreased tillage, integrating animal and plant agriculture, cooperating with nearby farms and towns, etc.) are used to lessen nutrient flows off the farm and until farms obtain more nutrients from local sources (legumes, leaves, composts, collected kitchen wastes, manures, clean sludges [biosolids]).
Regarding environmental losses, it is commonly assumed that the use of organic nutrient sources always results in lower impacts. This is only true if good management practices are followed. A study in Sweden compared conventional and organic crop production and found similar nitrate leaching losses. For example, in temperate climates a plowed alfalfa sod or a large manure application releases a lot of inorganic nitrogen that can easily meet all the needs of the following corn crop. However, if alfalfa is plowed too early—for example, in the early fall—much of the organic N is mineralized in the following months when the soil is still warm and can be lost through leaching or denitrification over the winter and spring. In this case N losses might be as high as when N fertilizer is applied too early. Organic sources may also create a problem with nutrient runoff if left on the surface, or with leaching when applied out of sync with plant uptake. While using organic nutrient sources has greater benefits for soil health than commercial fertilizers, the environmental impacts in both cases are best addressed through good agronomic management including 4R practices and careful consideration of environmental impacts.
Organic sources of nutrients have many other good qualities. Compared to commercial fertilizers that only “feed the plants,” organic materials also “feed the soil,” increasing biological activity by providing soil organisms with sources of energy as well as nutrients. Aggregates and humus are formed as organisms use the added organic materials. Organic sources can provide a more slow-release source of fertility, and the N availability better coincides with the needs of growing plants. Sources like manures or crop residues commonly contain all the needed nutrients, including the micronutrients, but they may not be present in the proper proportion for a particular soil and crop; thus, routine soil testing is important. Poultry manure, for example, has about the same levels of N and P, but plants take up three to five times more N than P. Applying it based on N needs of plants will therefore load the soil with unneeded P, increasing the pollution potential of any runoff. A lot of N is commonly lost during the composting process, making the compost much richer in P relative to N. Thus, applying a large quantity of compost to a soil that has sufficient P might supply a crop’s N needs but enriches the soil in unneeded P, creating a greater pollution potential.
One of the drawbacks to organic materials is the variable amounts and uncertain timing of nutrient release for plants to use. The value of manure as a nutrient source depends on the type of animal, its diet, and manure handling and application. For cover crops, the N contribution depends on the species, the amount of growth in the spring and the weather. In addition, manures typically are bulky and may contain a high percentage of water, so considerable effort is required to apply them per unit of nutrients. The timing of nutrient release is uncertain because it depends both on the type of organic materials used and the action of soil organisms. Their activities change with temperature and rainfall. Finally, the relative nutrient concentrations for a particular manure may not match soil needs. For example, manures may contain high amounts of both N and P when your soil already has high P levels.
UNDERSTANDING THE TERMS: ORGANIC FARMING VERSUS ORGANIC NUTRIENT SOURCES
For some, there is confusion around the term “organic.” We have used the term “organic sources” of nutrients to refer to nutrients contained in crop residues, manures and composts—i.e., the nutrients are applied in organic forms. All farmers, “conventional” and “organic,” use these types of materials. Both also use limestone and a few other materials. However, most of the commercial fertilizers listed in Table 18.2 are not allowed in organic production because they are synthetically derived. In place of sources such as urea, anhydrous ammonia, diammonium phosphate, concentrated superphosphate and muriate of potash, organic farmers use products that come directly from minerals, such as greensand, granite dust and rock phosphate. Other organic products come from parts of organisms, such as bone meal, fish meal, soybean meal and blood meal (see Table 18.3). Finally, to make matters more confusing, many countries, especially in Europe, label products as “bio” or “biological” when they are grown using organic practices.
Selection of Commercial Fertilizer Sources
There are numerous forms of commercial fertilizers given in Table 18.2. When you buy fertilizers in large quantities, you usually choose the cheapest source. When you buy bulk blended fertilizer, you usually don’t know what sources were used unless you ask. All you know is that it’s a 10-20-20 or a 20-10-10 (both referring to the percent of available N, P2O5 and K2O) or another blend. However, below is a number of examples of situations in which you might not want to apply the cheapest source.
- Although the cheapest N form is anhydrous ammonia, the problems with injecting it into a soil with many large stones or the losses that might occur if you inject it into very moist clay or dry sandy soil may call for other N sources to be used instead.
- If both N and P are needed, diammonium phosphate (DAP) is a good choice because it has approximately the same cost and P content as concentrated superphosphate and also contains 18% N.
- Although muriate of potash (potassium chloride) is the cheapest K source, it may not be the best choice under certain circumstances. If you also need magnesium and don’t need to lime the field, potassium magnesium sulfate would be a better choice.
The choice of fertilizer should be based on the nutrient needs of the crop and their availability in the soil (ideally determined by a soil test). However, the availability of the right fertilizer source may depend on the region. In countries with sophisticated agricultural supply infrastructures (like North America and Europe), farmers have a lot of choices of fertilizer materials and blends that match their needs. But in many developing countries fertilizer markets are underdeveloped and products are more expensive due to high transportation costs. (A 2011 study found fertilizer prices in Sub-Saharan Africa to be four times higher than in Europe). This limits the choices of fertilizer materials, and oftentimes farmers use only one or two fertilizer types (like DAP) without knowing the true crop needs.
| Table 18.3 Products Used by Organic Growers to Supply Nutrients | |||
|---|---|---|---|
| % N | % P2O5 | % K2O | |
| Alfalfa pellets | 2.7 | 0.5 | 2.8 |
| Blood meal | 13 | 2 | — |
| Bone meal | 3 | 20 | 0.5 |
| Cocoa shells | 1 | 1 | 3 |
| Colloidal phosphate | — | 18 | — |
| Compost | 1 | 0.4 | 3 |
| Cottonseed meal | 6 | 2 | 2 |
| Fish scraps, dried and ground | 9 | 7 | — |
| Granite dust | — | — | 5 |
| Greensand | — | — | 7 |
| Hoof and horn meal | 11 | 2 | — |
| Linseed meal | 5 | 2 | 1 |
| Rock phosphate | — | 30 | — |
| Seaweed, ground | 1 | 0.2 | 2 |
| Soybean meal | 6 | 1.4 | 4 |
| Tankage | 6.5 | 14.5 | — |
| Feather meal | 11-13 | — | — |
| Notes: 1. Values of P2O5 and K2O represent total nutrients present. For fertilizers listed in Table 18.2, the numbers are the amount that are readily available. 2. Organic growers also use potassium magnesium sulfate (“sul-po-mag” or “K-mag”), wood ashes, limestone and gypsum (listed in Table 18.2). Although some use only manure that has been composted, others will use aged manures (see Chapter 12). There are also a number of commercial organic products with a variety of trade names. (See materials listed by the Organic Materials Review Institute (OMRI) at www.omri.org.) Source: R. Parnes (1990) | |||
Method and Timing of Application
Fertilizer application timing and application methods are frequently related, so in this section both will be reviewed together.
Broadcast fertilizer application is evenly distributed over the whole field using a spin applicator (for granules) or sprayer (for liquids). If using plow or harrow tillage, it would usually be incorporated during tillage. Broadcasting is best used to increase the nutrient level of the bulk of the soil. It is especially useful to build P and K when they are very deficient. When using no-till, nutrients tend to be more stratified and care should be taken to lessen potential runoff that would be enriched in phosphorus—routine cover cropping will especially help. Broadcasting (with or without incorporation) usually occurs in the fall or in spring just before tillage. Broadcasting on top of a growing crop, called topdressing, is commonly used to apply N, especially to crops that occupy the entire soil surface, such as wheat or a grass hay crop. (Amendments used in large quantities, like lime and gypsum, are also broadcast over the soil surface.)
There are various methods of applying localized placement of fertilizer. Liquid nitrogen is often injected into the soil in bands because it reduces the potential for losses. Banding smaller amounts of fertilizer to the side and below the seed (usually two inches away) at planting is also a common application method. It is especially useful for row crops grown in cool soil conditions—early in the season, for example—on soils with high amounts of surface residues, with no-till management, or on wet soils that are slow to warm in the spring. It is also useful for soils that test low to medium (or even higher) in P and K. Band placement of fertilizer near the seed at planting, usually called starter fertilizer, may be a good idea even in warmer climates when planting early. It still might be cool enough to slow root growth and release of nutrients from organic matter. Including N as part of the starter fertilizer appears to help roots use fertilizer P more efficiently, perhaps because N stimulates root growth. Starter fertilizer for soils very low in fertility frequently contains other nutrients, such as sulfur, zinc, boron or manganese. While liquid starter fertilizer applied along with the seed at planting has proven successful in no-till planting of small grains, nitrogen rates need to be matched to soil type and planter type, and to row and seed spacing to avoid salt or ammonia damage.
Splitting N applications is a good management practice, especially on sandy soils where nitrate is easily lost by leaching, or on heavy loams and clays, where it can be lost by denitrification. Some N can be applied before planting or in the starter fertilizer band, and the rest can be applied as a sidedress or topdress during the growing season. In almost all situations sidedressing a good portion of needed N fertilizer is recommended for efficient use. However, this can increase the risk of reduced yields if the weather is too wet to apply the fertilizer (and you haven’t put on enough N in a preplant or starter application) or is too dry following an application. In the latter case the fertilizer stays on the surface instead of washing into the root zone. Although unusual nationally, recommendations for split K applications are made for very sandy soils with low organic matter, such as on Georgia’s coastal plain, especially if there has been enough rainfall to cause K to leach into the subsoil. Almost all commercial vegetable farmers use irrigation and can easily apply fertilizer through the watering system during the season (this is called “fertigation”). This is especially attractive with drip irrigation, which allows spoon feeding of the crop to maximize nutrient uptake efficiencies. Fertigation of agronomic row crops is common in some regions, frequently by center pivot systems.
CROP VALUE, FERTILIZER COST AND FERTILIZER RATES
Most agronomic crops grown on large acreages are worth around $400–$1,000 per acre, and the fertilizer used may represent 25% of non-land growing costs. So, if a corn farmer uses 100 pounds of N that’s not needed (at about $40), that may represent 5% or more of gross income. Add in some unneeded P and K and the implications for lost net revenue become clear. Some years ago, one of the authors of this book worked with two brothers who operated a dairy farm in northern Vermont that had high soil test levels of N, P and K. Despite his recommendation of no fertilizer, the normal practice was followed, and N, P and K fertilizer worth $70 per acre (in 1980s prices) was applied to their 200 acres of corn. The yields on 40-foot-wide, no-fertilizer strips that they left in each field were the same as where fertilizer had been applied, so while some of the P and K might be available to crops in future years, the $14,000 they spent on fertilizer was mostly wasted.
When growing fruit or vegetable crops worth thousands of dollars per acre, fertilizers represent about 1% of the value of the crop and 2% of the costs. But when growing specialty crops (medicinal herbs, certain organic vegetables for direct marketing) worth over $10,000 per acre, fertilizer costs are dwarfed by other costs, such as hand labor. A waste of $70 per acre in unneeded nutrients for these crops would cause a minimal economic penalty, assuming you maintain a reasonable balance between nutrients, but there may be environmental and crop quality reasons against applying too much fertilizer. In general, relative nutrient expenses are greatest for the low-value crops, but these are also grown on the most extensive acres where cumulatively they have the biggest environmental impacts.
FERTILIZER GRADE: OXIDE VERSUS ELEMENTAL FORMS
When talking or reading about fertilizer P or K, the oxide forms are used. They are also used in all recommendations and when you buy fertilizer. The terms “phosphate” (P2O5) and “potash” (K2O) have been used for so long to refer to phosphorus and potassium in fertilizers, it is likely that they will be with us indefinitely, even if they are confusing. In fact, their use is codified in state regulations in the United States and by national regulations in Canada. When you apply 100 pounds of potash per acre, you actually apply 100 pounds of K2O: the equivalent of 83 pounds of elemental potassium. Of course, you are really not using K2O but are rather using something like muriate of potash (KCl). It’s similar with phosphate—100 pounds of P2O5 per acre is the same as 44 pounds of P—and you’re really using fertilizers like concentrated superphosphate (that contains a form of calcium phosphate) or ammonium phosphate. However, in your day-to-day dealing with fertilizers you need to think in terms of nitrogen, phosphate and potash, and not in actual amounts of elemental P or K you purchase or apply.
Tillage and Fertility Management: To Incorporate or Not?
It is possible to incorporate fertilizers and amendments with systems that provide some tillage, such as moldboard plow and harrow, disk harrow alone, chisel plow, zone/strip-till and ridge-till. However, when using pure no-till production systems, it is not possible to mix fertilizer materials into the soil to uniformly raise the fertility level in that portion of the soil where roots are especially active. However, surface-applied fertilizers in no-till systems usually work their way down to the upper part of the root zone.
When broadcasting fertilizer without incorporation, as occurs with no-till, there are potential losses that can occur. For example, significant quantities of ammonia may be lost by volatilization when the most commonly used solid N fertilizer, urea, is left on the soil surface. Thus if rainfall isn’t going to occur very soon after application, another solid source of N fertilizer or a liquid fertilizer should be used. Also, nutrients remaining on the surface after application are much more likely to be lost in runoff during rain events. Although the amount of runoff is usually lower with reduced tillage systems than with conventional tillage, the concentration of nutrients in the runoff may be quite a bit higher. This makes using cover crops as a routine management practice even more important. Over time, using no-till and cover crops, rainfall infiltration rates tend to increase, lessening runoff.
A special concern exists with heavy clay soils that develop continuous macropores from cracks and biological activity (especially deep-burrowing earthworms). Although this is generally good for the health of the soil and crop growth, it can also pose concerns with fertilizer and manure applications when the soils have subsurface (tile) drainage. When materials applied on the soil surface are not incorporated, nutrients can readily enter the macropores with heavy rains, move rapidly to the tile lines and then discharge into waterways.
SOIL TESTS
Routine soil tests, one of the key nutrient management tools, are discussed in detail in Chapter 21. For newer soil health tests see Chapter 23.
If you are thinking about changing from conventional tillage to no-till or other forms of reduced tillage, incorporate needed lime, phosphate and potash, as well as manures and other organic residues, before making the switch. It’s your last chance to easily change the fertility of the top 8 or 9 inches of soil.
Chapter 18 Sources
Gregory, D.L. and B.L. Bumb. 2006. Factors Affecting Supply of Fertilizer in Sub-Saharan Africa. Agric. Rural Develp. Disc. Paper 24. World Bank.
Mikkelsen, R. and T.K. Hartz. 2008. Nitrogen sources for organic crop production. Better Crops 92(4): 16–19.
OMAFRA (Ontario Ministry of Agriculture, Food, and Rural Affairs). 1997. Nutrient Management. Best Management Practices Series. Available from the Ontario Federation of Agriculture, Toronto, Ontario, Canada.
Parnes, R. 1990. Fertile Soil: A Grower’s Guide to Organic and Inorganic Fertilizers. agAccess: Davis, CA.
The Fertilizer Institute. 2020. What are the 4Rs? https://nutrientstewardship.org/4rs/
Torstensson, G., H. Aronsson and L. Bergstrom. 2006. Nutrient use efficiencies and leaching of organic and conventional cropping systems in Sweden. Agronomy Journal 98: 603–615. van Es, H.M., K.J. Czymmek and Q.M. Ketterings. 2002. Management effects on N leaching and guidelines for an N leaching index in New York. Journal of Soil and Water Conservation 57(6): 499–504.
